Learning Outcomes for Chemical Reactions and
... 6. Uses of acids in food and drink and their impact on health. 7. Acids contain aqueous hydrogen ions, H+(aq). 8. Dissociation of water into hydrogen and hydroxide ions. 9. pH is related to the concentration of hydrogen and hydroxide ions in pure water, acids and alkalis. 10. Acids are corrosive and ...
... 6. Uses of acids in food and drink and their impact on health. 7. Acids contain aqueous hydrogen ions, H+(aq). 8. Dissociation of water into hydrogen and hydroxide ions. 9. pH is related to the concentration of hydrogen and hydroxide ions in pure water, acids and alkalis. 10. Acids are corrosive and ...
NAME…………… - Kcse Online
... In a separate experiment the student reacted iron and hydrochloric acid to prepare hydrogen gas. (i) Write an ionic equation for the reaction. (1mk) ...
... In a separate experiment the student reacted iron and hydrochloric acid to prepare hydrogen gas. (i) Write an ionic equation for the reaction. (1mk) ...
AP Ch. 20 Notes (2005)
... has a lower electrical potential energy than the anode. • Potential difference: difference in electrical potential. • The potential difference is measured in volts… One volt (V) is the potential difference required to impart one joule (J) of energy to a charge of one coulomb (C). ...
... has a lower electrical potential energy than the anode. • Potential difference: difference in electrical potential. • The potential difference is measured in volts… One volt (V) is the potential difference required to impart one joule (J) of energy to a charge of one coulomb (C). ...
CHEM 101 1st Major (Term 161)
... D) It is a strong electrolyte. E) It produces H+ and NO3- in aqueous solution. ...
... D) It is a strong electrolyte. E) It produces H+ and NO3- in aqueous solution. ...
A-level Paper 1 Practice Paper 8 - A
... A rechargeable nickel–cadmium cell is an alternative to the cell shown in part (c). The relevant half-equations for this cell are equations 1 and 3 in the table above. (i) ...
... A rechargeable nickel–cadmium cell is an alternative to the cell shown in part (c). The relevant half-equations for this cell are equations 1 and 3 in the table above. (i) ...
the nakuru district sec. schools trial examinations - 2015
... ∆ H = -97kj/mol (a) Name one source of hydrogen gas used in this process (1 mark) Cracking of long chained alkanes – natural gas or coal natural gas electrolysis of brine (b) Name the catalyst used in the above reaction ...
... ∆ H = -97kj/mol (a) Name one source of hydrogen gas used in this process (1 mark) Cracking of long chained alkanes – natural gas or coal natural gas electrolysis of brine (b) Name the catalyst used in the above reaction ...
What is Matter PowerPoint
... Mixture of iodine solid and sodium chloride (Hint: Iodine is not soluble in water) Hydrogen from oxygen in water Mixture of lead and aluminum Mixture of salt and iron filings Sodium from chlorine in salt ...
... Mixture of iodine solid and sodium chloride (Hint: Iodine is not soluble in water) Hydrogen from oxygen in water Mixture of lead and aluminum Mixture of salt and iron filings Sodium from chlorine in salt ...
1.5.16(Chem) - mrcarlsonschemistryclass
... • Atoms bonded together with an IONIC bond are called ionic compounds. • An ionic bond is a METAL bonded with a NONMETAL. • Draw the crystal lattice structure for sodium chloride: ...
... • Atoms bonded together with an IONIC bond are called ionic compounds. • An ionic bond is a METAL bonded with a NONMETAL. • Draw the crystal lattice structure for sodium chloride: ...
Bio 102 Lecture - chapter 2 The Chemical Basis of Life
... Attraction of oppositely charged ions holds the two atoms together in an ionic bond. ...
... Attraction of oppositely charged ions holds the two atoms together in an ionic bond. ...
Review redox reactions
... Iodide will react with permanganate ions to form iodine and manganese (IV) oxide. Write the balanced net ionic equation if the reaction occurs in an acidic solution. ...
... Iodide will react with permanganate ions to form iodine and manganese (IV) oxide. Write the balanced net ionic equation if the reaction occurs in an acidic solution. ...
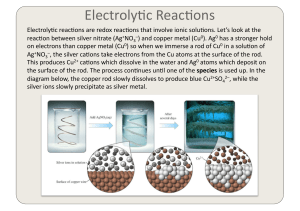
Electrochemistry 2
... reac)on between silver nitrate (Ag+NO3–) and copper metal (Cu0). Ag0 has a stronger hold on electrons than copper metal (Cu0) so when we immerse a rod of Cu0 in a solu)on of Ag+NO3–, the silver ...
... reac)on between silver nitrate (Ag+NO3–) and copper metal (Cu0). Ag0 has a stronger hold on electrons than copper metal (Cu0) so when we immerse a rod of Cu0 in a solu)on of Ag+NO3–, the silver ...
honors final key
... 8. List five indicators of a chemical change a. Bubbling b. Temperature change c. Precipitate d. Color change e. Mass change 9. What is the only way to determine that a chemical reaction has taken place? Can not be undone 10. Give the general format for a chemical reaction. Reactants products 11. ...
... 8. List five indicators of a chemical change a. Bubbling b. Temperature change c. Precipitate d. Color change e. Mass change 9. What is the only way to determine that a chemical reaction has taken place? Can not be undone 10. Give the general format for a chemical reaction. Reactants products 11. ...
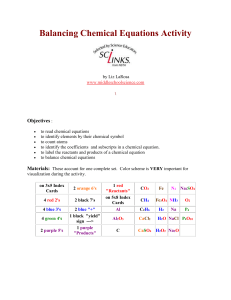
Balancing Chemical Equations Activity by Liz LaRosa www
... Print activity cards on card stock instead of making index cards for quicker set up. The color coding is very important for visualization. It is easier and quicker to locate the elements that you are trying to balance. If everything is in black ink, its harder to distinguish the equation contents. I ...
... Print activity cards on card stock instead of making index cards for quicker set up. The color coding is very important for visualization. It is easier and quicker to locate the elements that you are trying to balance. If everything is in black ink, its harder to distinguish the equation contents. I ...
Writing and Balancing Chemical Equations
... arrow (→) separates the reactants from the products (arrow points to products) –Read as: “reacts to form” or yields The plus sign = “and” (s) after the formula = solid: Fe(s) (g) after the formula = gas: CO2(g) (l) after the formula = liquid: H2O(l) ...
... arrow (→) separates the reactants from the products (arrow points to products) –Read as: “reacts to form” or yields The plus sign = “and” (s) after the formula = solid: Fe(s) (g) after the formula = gas: CO2(g) (l) after the formula = liquid: H2O(l) ...
Chemistry B1A - Bakersfield College
... explain what would happen if you did the following: a. First you drop a plastic bead that has a density of 0.24 g/cm3 into the column. b. You drop a bead in that makes it all the way to the bottom. What can you say about the density of this bead? c. You drop a bead with a volume of 0.043 mL and a ma ...
... explain what would happen if you did the following: a. First you drop a plastic bead that has a density of 0.24 g/cm3 into the column. b. You drop a bead in that makes it all the way to the bottom. What can you say about the density of this bead? c. You drop a bead with a volume of 0.043 mL and a ma ...
Glossary - WordPress.com
... The formula of a compound which shows the minimum ratio present between the atoms. Electron Affinity The amount of energy given out when an electron is absorbed in the outermost electronic shell of all isolated gaseous atom. Its units are KJ/mol. Electro-Negativity It is the power of an atom to attr ...
... The formula of a compound which shows the minimum ratio present between the atoms. Electron Affinity The amount of energy given out when an electron is absorbed in the outermost electronic shell of all isolated gaseous atom. Its units are KJ/mol. Electro-Negativity It is the power of an atom to attr ...
Atomic Theories and Models - MrD-Home
... version of the _________ element (isotope) will be produced. • e.g. ...
... version of the _________ element (isotope) will be produced. • e.g. ...
File
... A reversible reaction. The reaction can occur in both directions. Acetic acid is a weak electrolyte because its ionization in water is incomplete. ...
... A reversible reaction. The reaction can occur in both directions. Acetic acid is a weak electrolyte because its ionization in water is incomplete. ...
IB Chemistry Brakke ECA - Topic 15 T15D12
... Calculate ∆G for the reaction at 238 K. State and explain whether the reaction is spontaneous. ...
... Calculate ∆G for the reaction at 238 K. State and explain whether the reaction is spontaneous. ...
1 - UCSB C.L.A.S.
... 4. Consider the reaction: CaCl2(s) → Ca2+(aq) + 2Cl-(aq) ΔH = -81.5 kJ If 20.0 g of calcium chloride are dissolved in 150 mL of water at 25.0 C, what will be the final temperature of the solution assuming no heat loss to the surroundings? 5. Define the following and draw energy diagrams to represent ...
... 4. Consider the reaction: CaCl2(s) → Ca2+(aq) + 2Cl-(aq) ΔH = -81.5 kJ If 20.0 g of calcium chloride are dissolved in 150 mL of water at 25.0 C, what will be the final temperature of the solution assuming no heat loss to the surroundings? 5. Define the following and draw energy diagrams to represent ...
Electrolysis of water
Electrolysis of water is the decomposition of water (H2O) into oxygen (O2) and hydrogen gas (H2) due to an electric current being passed through the water.This technique can be used to make hydrogen fuel (hydrogen gas) and breathable oxygen; though currently most industrial methods make hydrogen fuel from natural gas instead.