File
... Reactions in which one or more electrons are transferred are called oxidation-reduction reactions or redox reactions. Half reaction- one involving oxidation and the other involving reduction For acidic solution 1. Write separate equations for oxidation and reduction half reactions 2. For each half-r ...
... Reactions in which one or more electrons are transferred are called oxidation-reduction reactions or redox reactions. Half reaction- one involving oxidation and the other involving reduction For acidic solution 1. Write separate equations for oxidation and reduction half reactions 2. For each half-r ...
Unit 3: States of Matter Review
... 1. What simple evidence demonstrates that gas particles are in motion? 2. Why are there no minus numbers on the Kelvin scale? 3. The boiling point of water in city A is 94°C; in city B it is 97°C. Which city is at the higher elevation? 4. Explain why it would be difficult to cook an egg to a hard-bo ...
... 1. What simple evidence demonstrates that gas particles are in motion? 2. Why are there no minus numbers on the Kelvin scale? 3. The boiling point of water in city A is 94°C; in city B it is 97°C. Which city is at the higher elevation? 4. Explain why it would be difficult to cook an egg to a hard-bo ...
2016 - Specimen Paper 2 - Cambridge International Examinations
... Permission to reproduce items where third-party owned material protected by copyright is included has been sought and cleared where possible. Every reasonable effort has been made by the publisher (UCLES) to trace copyright holders, but if any items requiring clearance have unwittingly been included ...
... Permission to reproduce items where third-party owned material protected by copyright is included has been sought and cleared where possible. Every reasonable effort has been made by the publisher (UCLES) to trace copyright holders, but if any items requiring clearance have unwittingly been included ...
Unit 3 Test - hrsbstaff.ednet.ns.ca
... ___ Combustibility is the ability of a substance to react with acids ___ Sugar disappearing in water is an example of a solution ___ Raisins in Raisin Bran are an example of a solution ___ Lighting a test tube of acetylene gas is an example of a reaction with acid ___ Lighting a test tube of acetyle ...
... ___ Combustibility is the ability of a substance to react with acids ___ Sugar disappearing in water is an example of a solution ___ Raisins in Raisin Bran are an example of a solution ___ Lighting a test tube of acetylene gas is an example of a reaction with acid ___ Lighting a test tube of acetyle ...
AP_chemistry_Summer_Assignment_2014
... 61.When Hydrogen sulfide gas, H2S, reacts with oxygen, Sulfur dioxide gas and steam are produced. a.Write the balanced chemical equation for this reaction. b.How many liters of sulfur dioxide would be produced from 4.0 l of Oxygen? Assume 100% yield and that all gases are measured at the same temper ...
... 61.When Hydrogen sulfide gas, H2S, reacts with oxygen, Sulfur dioxide gas and steam are produced. a.Write the balanced chemical equation for this reaction. b.How many liters of sulfur dioxide would be produced from 4.0 l of Oxygen? Assume 100% yield and that all gases are measured at the same temper ...
Semester 2 review questions
... 6. The theory that no two electrons in an atom can share the same 4 quantum numbers. 7. The theory that electrons fill the lowest energy orbitals first. 8. The theory that, within a sublevel, electrons prefer to occupy their own orbital. 9. A term describing the outermost electrons in an atom. 10. A ...
... 6. The theory that no two electrons in an atom can share the same 4 quantum numbers. 7. The theory that electrons fill the lowest energy orbitals first. 8. The theory that, within a sublevel, electrons prefer to occupy their own orbital. 9. A term describing the outermost electrons in an atom. 10. A ...
chapter 1 - Revsworld
... (18) A certain element has two naturally occurring isotopes. These isotopes have mass numbers of 63 and 65, and their fractional abundances are, respectively, 0.692 (69.2%) and 0.308 (30.8%). What is the atomic weight (or atomic mass) of this element? a) b) c.) d) e) ...
... (18) A certain element has two naturally occurring isotopes. These isotopes have mass numbers of 63 and 65, and their fractional abundances are, respectively, 0.692 (69.2%) and 0.308 (30.8%). What is the atomic weight (or atomic mass) of this element? a) b) c.) d) e) ...
final exam review packet
... 41. When a substance dissolves in water: A. The solution’s ___________________ point is higher. (when you cook pasta) B. The solution’s ___________________ point is lower. (when you add salt to roads in winter) C. These properties are called __________________properties because they depend on the c ...
... 41. When a substance dissolves in water: A. The solution’s ___________________ point is higher. (when you cook pasta) B. The solution’s ___________________ point is lower. (when you add salt to roads in winter) C. These properties are called __________________properties because they depend on the c ...
Chemistry Spring Final Review
... D. 192 18. Given the reaction: __Mg + __HCl __MgCl 2 + __H2 , what is the total number of grams of Mg consumed when 0.50 mole of H2 is produced? A. 6.0 g B. 12 g C. 3.0 g D. 24 g 19. What is the correct coefficient for carbon monoxide in the reaction Fe 2 O 3 + CO Fe + CO 2 ? A. 6 B. 3 C. 5 D. 1 ...
... D. 192 18. Given the reaction: __Mg + __HCl __MgCl 2 + __H2 , what is the total number of grams of Mg consumed when 0.50 mole of H2 is produced? A. 6.0 g B. 12 g C. 3.0 g D. 24 g 19. What is the correct coefficient for carbon monoxide in the reaction Fe 2 O 3 + CO Fe + CO 2 ? A. 6 B. 3 C. 5 D. 1 ...
Unit 2
... Welcome to Advanced Placement Chemistry. AP Chem is a fast paced course, with higher orders of thinking. You will be expected to pull on previous knowledge constantly to solve problems. Along the way, we will see some fun demonstrations and perform some intense chemical experiments. The attached sum ...
... Welcome to Advanced Placement Chemistry. AP Chem is a fast paced course, with higher orders of thinking. You will be expected to pull on previous knowledge constantly to solve problems. Along the way, we will see some fun demonstrations and perform some intense chemical experiments. The attached sum ...
Unit 2
... Welcome to Advanced Placement Chemistry. AP Chem is a fast paced course, with higher orders of thinking. You will be expected to pull on previous knowledge constantly to solve problems. Along the way, we will see some fun demonstrations and perform some intense chemical experiments. The attached sum ...
... Welcome to Advanced Placement Chemistry. AP Chem is a fast paced course, with higher orders of thinking. You will be expected to pull on previous knowledge constantly to solve problems. Along the way, we will see some fun demonstrations and perform some intense chemical experiments. The attached sum ...
Preview Sample 1
... D) are always some form of carbohydrate. E) are naturally similar to sugars. 102) Alaska Natives have a lower incidence of heart disease even though their diets are high in fat and cholesterol. This may be due to the large amount of ________ in their diets. A) steroids B) omega-3 fatty acids C) trig ...
... D) are always some form of carbohydrate. E) are naturally similar to sugars. 102) Alaska Natives have a lower incidence of heart disease even though their diets are high in fat and cholesterol. This may be due to the large amount of ________ in their diets. A) steroids B) omega-3 fatty acids C) trig ...
Chapter 4
... concentration until the chemical reaction between the two solutions is complete. Equivalence point – the point at which the reaction is complete Indicator – substance that changes color at (or near) the ...
... concentration until the chemical reaction between the two solutions is complete. Equivalence point – the point at which the reaction is complete Indicator – substance that changes color at (or near) the ...
General Chemistry Review Problems
... b. If 4 moles of LiOH reacts, how many moles of water will be produced? c. How many liters of carbon dioxide are required to completely react with 45 grams of lithium hydroxide at STP? d. How many grams of lithium hydroxide is required to produce 25 g of lithium carbonate? e. How many moles of water ...
... b. If 4 moles of LiOH reacts, how many moles of water will be produced? c. How many liters of carbon dioxide are required to completely react with 45 grams of lithium hydroxide at STP? d. How many grams of lithium hydroxide is required to produce 25 g of lithium carbonate? e. How many moles of water ...
chapter_2_2009
... The atoms sharing electrons sit close enough together so that their outer energy levels overlap. Single covalent bond-one pair of electrons is shared. ...
... The atoms sharing electrons sit close enough together so that their outer energy levels overlap. Single covalent bond-one pair of electrons is shared. ...
PRACTICE * Naming and Writing Ionic Compounds
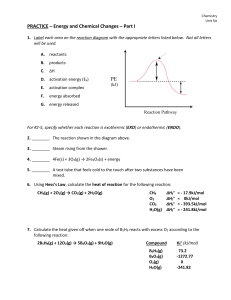
... 1. Label each area on the reaction diagram with the appropriate letters listed below. Not all letters will be used. A. ...
... 1. Label each area on the reaction diagram with the appropriate letters listed below. Not all letters will be used. A. ...
chapter_2_2007
... Atoms can gain or lose electrons to achieve a full outermost energy level. – Atoms with charge are called ions. – When an atom gives away an electron, it ends up with more protons than electrons and gains a positive charge; cation – When an atom accepts an electron, it ends up with more electrons th ...
... Atoms can gain or lose electrons to achieve a full outermost energy level. – Atoms with charge are called ions. – When an atom gives away an electron, it ends up with more protons than electrons and gains a positive charge; cation – When an atom accepts an electron, it ends up with more electrons th ...
Practice Bypass Answers
... h) At room temperature (72 oF) propane is a gas and water is a liquid. This means that 72 oF must be higher than the boiling point for propane, but lower than the boiling point for water. Explain why propane has a lower boiling point than water. Provide an analysis of the interparticle forces betwee ...
... h) At room temperature (72 oF) propane is a gas and water is a liquid. This means that 72 oF must be higher than the boiling point for propane, but lower than the boiling point for water. Explain why propane has a lower boiling point than water. Provide an analysis of the interparticle forces betwee ...
S3 Chemistry - eduBuzz.org
... State the location, charge and mass of each sub atomic particle Calculate the number of protons, neutrons and electrons in an atom Identify whether a species has an equal or unequal number of protons and electrons and use this to state whether it is an atom or ion. State the charge of an ion ...
... State the location, charge and mass of each sub atomic particle Calculate the number of protons, neutrons and electrons in an atom Identify whether a species has an equal or unequal number of protons and electrons and use this to state whether it is an atom or ion. State the charge of an ion ...
A simple calorimeter was used as a vessel to measure the heat
... A simple calorimeter was used as a vessel to measure the heat evolved or absorbed during the following reaction: ...
... A simple calorimeter was used as a vessel to measure the heat evolved or absorbed during the following reaction: ...
File
... Chemical Change • The way to tell if a chemical change is occurring or has occurred is if there is a new substance produced, accompanied by a change in colour, odour, state, or energy. • Changes in state usually involve formation of gas or solid. ...
... Chemical Change • The way to tell if a chemical change is occurring or has occurred is if there is a new substance produced, accompanied by a change in colour, odour, state, or energy. • Changes in state usually involve formation of gas or solid. ...
Electrolysis of water
Electrolysis of water is the decomposition of water (H2O) into oxygen (O2) and hydrogen gas (H2) due to an electric current being passed through the water.This technique can be used to make hydrogen fuel (hydrogen gas) and breathable oxygen; though currently most industrial methods make hydrogen fuel from natural gas instead.