Chapter 4 - Aqueous Reactions
... Note that the equation is balanced for both mass and charge!!! ...
... Note that the equation is balanced for both mass and charge!!! ...
AP Chemistry Predicting Products Tutorial
... 4H+ + SO42- + Ba2+ + 2OH- BaSO4 + 2H2O 2. A solution of sodium hydroxide is added to a solution of sodium dihydrogen phosphate until the same number of moles of each compound has been added. OH- + H2PO4- HOH + HPO42(Note: H2PO4- can act as an acid to donate an H+ and become HPO42- or it can act ...
... 4H+ + SO42- + Ba2+ + 2OH- BaSO4 + 2H2O 2. A solution of sodium hydroxide is added to a solution of sodium dihydrogen phosphate until the same number of moles of each compound has been added. OH- + H2PO4- HOH + HPO42(Note: H2PO4- can act as an acid to donate an H+ and become HPO42- or it can act ...
4.5 Solid fast-ion conductors 1
... Gauzes have platinum wires welded to them which are connected to a high-impedance voltmeter Platinum catalyses the dissociation and recombination of oxygen molecules so that O2ions can be formed at one electrode & converted Fig. 4.28 Schematic diagram of a solid electrolyte oxygen probe suitable for ...
... Gauzes have platinum wires welded to them which are connected to a high-impedance voltmeter Platinum catalyses the dissociation and recombination of oxygen molecules so that O2ions can be formed at one electrode & converted Fig. 4.28 Schematic diagram of a solid electrolyte oxygen probe suitable for ...
Matter is anything that occupies space and has mass. Examples
... Specific heat – the amount of heat necessary to change 1 gram of a substance by 1 OC. Water has one of the highest specific heats. It takes a long time to heat up and it is slow to cool down. Air will expand when it is warm and this causes the wind to blow from the warmer area to the cooler area. ...
... Specific heat – the amount of heat necessary to change 1 gram of a substance by 1 OC. Water has one of the highest specific heats. It takes a long time to heat up and it is slow to cool down. Air will expand when it is warm and this causes the wind to blow from the warmer area to the cooler area. ...
experiment 7 - (canvas.brown.edu).
... 2. The overall hydrogen fuel cell reaction is H2 (g)+1/2 O2 (g) H2O (l). Write down the two half reactions and calculate the standard fuel cell potential, Eo (or open circuit potential), using their standard half cell reduction potentials (See Zumdahl Appendix five, or Tro Appendix II D). ...
... 2. The overall hydrogen fuel cell reaction is H2 (g)+1/2 O2 (g) H2O (l). Write down the two half reactions and calculate the standard fuel cell potential, Eo (or open circuit potential), using their standard half cell reduction potentials (See Zumdahl Appendix five, or Tro Appendix II D). ...
1. When the reaction Cu + HNO3 → Cu2+ + NO + H2O is balanced
... 50. In an electrolysis experiment 1.44 g of Ag was deposited from an aqueous AgNO3 solution, while 0.120 g of the metal X was deposited in another cell that contains aqueous XCl3 (connected in series with the AgNO3 cell.) The molar mass of X is –––– g/mol. A) 63.3 B) 108 C) 27.0 D) 31.8 ...
... 50. In an electrolysis experiment 1.44 g of Ag was deposited from an aqueous AgNO3 solution, while 0.120 g of the metal X was deposited in another cell that contains aqueous XCl3 (connected in series with the AgNO3 cell.) The molar mass of X is –––– g/mol. A) 63.3 B) 108 C) 27.0 D) 31.8 ...
Intro to Chemical Equations note
... type appearing on both sides Balance the elements one at a time by adding coefficients (the numbers in front) - save H and O until LAST! Check to make sure it is balanced. ...
... type appearing on both sides Balance the elements one at a time by adding coefficients (the numbers in front) - save H and O until LAST! Check to make sure it is balanced. ...
Chapter 23 (Section 3) Pregnancy, Birth, and Childhood (Pages 735
... 4. MOLECULE SMALLEST unit of a COMPOUND; maintaining PROPERTIES of the compound 5. ELEMENT matter that is composed of one kind of ATOM (e.g. sulfur [S]; carbon [C]) a. each ELEMENT has its own CHARACTERISTIC chemical and PHYSICAL properties *b. elements can NOT be BROKEN down into other substanc ...
... 4. MOLECULE SMALLEST unit of a COMPOUND; maintaining PROPERTIES of the compound 5. ELEMENT matter that is composed of one kind of ATOM (e.g. sulfur [S]; carbon [C]) a. each ELEMENT has its own CHARACTERISTIC chemical and PHYSICAL properties *b. elements can NOT be BROKEN down into other substanc ...
Chapter 8
... oxygen, releasing a large amount of energy in the form of light and heat. Reactive elements combine with oxygen ...
... oxygen, releasing a large amount of energy in the form of light and heat. Reactive elements combine with oxygen ...
Welcome to AP Chemistry!
... 5) When ammonia gas, oxygen gas and methane gas (CH4) are combined, the products are hydrogen cyanide gas and water. a. Write a balanced chemical equation for this reaction. b. Calculate the mass of each product produced when 225 g of oxygen gas is reacted with an excess of the other two reactants. ...
... 5) When ammonia gas, oxygen gas and methane gas (CH4) are combined, the products are hydrogen cyanide gas and water. a. Write a balanced chemical equation for this reaction. b. Calculate the mass of each product produced when 225 g of oxygen gas is reacted with an excess of the other two reactants. ...
Gas-forming Reactions
... more difficult to remove and the bisulfate ion is only partially ionized. The bisulfate ion is a weak acid. A base is a substance that increases the concentration of aqueous OH– ions when it is dissolved in water. Bases can be either ionic or molecular substances. A base can be thought of as a subst ...
... more difficult to remove and the bisulfate ion is only partially ionized. The bisulfate ion is a weak acid. A base is a substance that increases the concentration of aqueous OH– ions when it is dissolved in water. Bases can be either ionic or molecular substances. A base can be thought of as a subst ...
Electrochemistry
... the cathode, and they spontaneously flow through an external circuit from the anode to the cathode. ...
... the cathode, and they spontaneously flow through an external circuit from the anode to the cathode. ...
Chemistry- CST Review
... 2. Explain what you would do to quickly dissolve cube sugar in a cup of coffee (Like changes in temperature and surface area, breaking up the cube sugar). In order to dissolve a cube of sugar, you would increase surface area by breaking it up and increase temperature by heating the mixture. 3. What ...
... 2. Explain what you would do to quickly dissolve cube sugar in a cup of coffee (Like changes in temperature and surface area, breaking up the cube sugar). In order to dissolve a cube of sugar, you would increase surface area by breaking it up and increase temperature by heating the mixture. 3. What ...
2. Chemistry of Living Things Outline
... place more efficiently than they otherwise would at body temperature. For example, amino acids are produced from protein digestion. The enzymes needed for this reaction are not changed but must be present for the reaction to occur. Some enzymes have a __________________ part called a _______________ ...
... place more efficiently than they otherwise would at body temperature. For example, amino acids are produced from protein digestion. The enzymes needed for this reaction are not changed but must be present for the reaction to occur. Some enzymes have a __________________ part called a _______________ ...
Chemistry of Living Things Outline
... ______________ to take place more efficiently than they otherwise would at body temperature. For example, amino acids are produced from protein digestion. The enzymes needed for this reaction are not changed but must be present for the reaction to occur. Some enzymes have a __________________ pa ...
... ______________ to take place more efficiently than they otherwise would at body temperature. For example, amino acids are produced from protein digestion. The enzymes needed for this reaction are not changed but must be present for the reaction to occur. Some enzymes have a __________________ pa ...
Nature of Molecules and Water
... • Atoms shift from one molecule to another without any change in number or identity of atoms • Reactants = original molecules • Products = molecules resulting from reaction 6H2O + 6CO2 reactants ...
... • Atoms shift from one molecule to another without any change in number or identity of atoms • Reactants = original molecules • Products = molecules resulting from reaction 6H2O + 6CO2 reactants ...
11 BALANCING CHEMICAL EQUATIONS 1. 2 K + 1
... 1) Pick one reaction which made a Precipitate from each column and write the ionic reaction. 2) Use the solubility rule to determine which product formed the precipitate & which was soluble. 3) Cross out the products which were soluble because they’re spectators as reactants & products. ...
... 1) Pick one reaction which made a Precipitate from each column and write the ionic reaction. 2) Use the solubility rule to determine which product formed the precipitate & which was soluble. 3) Cross out the products which were soluble because they’re spectators as reactants & products. ...
Reactions of common metals and properties of
... Atoms of the alkali metals are easily excited; even the flame of a Bunsen burner can excite their valence electrons. As the electrons jump back to lower energy levels, they give characteristic colours to the flame; lithium imparts a red colour, sodium a yellow colour, and potassium a lilac colour. T ...
... Atoms of the alkali metals are easily excited; even the flame of a Bunsen burner can excite their valence electrons. As the electrons jump back to lower energy levels, they give characteristic colours to the flame; lithium imparts a red colour, sodium a yellow colour, and potassium a lilac colour. T ...
Synthesis of Alum Lab
... d) Alum forms from potassium ions, hydrated aluminum ions sulfate ions and water: K+(aq) + 6H2O(l) + 2SO42-(aq) + [Al(H2O)6]3+(aq) KAl(SO4)2.12H2O(s) ...
... d) Alum forms from potassium ions, hydrated aluminum ions sulfate ions and water: K+(aq) + 6H2O(l) + 2SO42-(aq) + [Al(H2O)6]3+(aq) KAl(SO4)2.12H2O(s) ...
Chemical Questions
... compressed air through a nozzle. • The water vapor expands as it passes through the nozzle and then freezes to a solid or snow. • Recall that when we heat a gas at constant T, its volume increases (expansion). We have to provide heat in this case. • If we insulate the gas from the heat source but st ...
... compressed air through a nozzle. • The water vapor expands as it passes through the nozzle and then freezes to a solid or snow. • Recall that when we heat a gas at constant T, its volume increases (expansion). We have to provide heat in this case. • If we insulate the gas from the heat source but st ...
Answer Key to Sample Questions
... positive because one molecule breaks to form two molecules b. What is the sign of H for this reaction? positive because a bond is broken, but none is formed. c. In which temperature range will this reaction be thermodynamically favored? It is entropy favored, enthalpy disfavored, so favored overall ...
... positive because one molecule breaks to form two molecules b. What is the sign of H for this reaction? positive because a bond is broken, but none is formed. c. In which temperature range will this reaction be thermodynamically favored? It is entropy favored, enthalpy disfavored, so favored overall ...
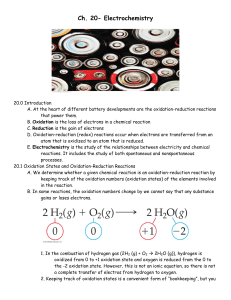
Ch. 20- Electrochemistry
... 1. Rusting of iron requires both oxygen and water, and the process can be accelerated by other factors such as pH, presence of salts, contact with metals more difficult to oxidize than iron, and stress on the iron. E. Preventing Corrosion of Iron 1. Objects made of iron are often covered with a coat ...
... 1. Rusting of iron requires both oxygen and water, and the process can be accelerated by other factors such as pH, presence of salts, contact with metals more difficult to oxidize than iron, and stress on the iron. E. Preventing Corrosion of Iron 1. Objects made of iron are often covered with a coat ...
FINAL REVIEW
... d) Write a balanced equation for the decomposition of calcium carbonate into calcium oxide and carbon dioxide. CaCO3 → CaO + CO2 decomposition/ analysis e) Write a balanced equation for the complete combustion of propane (C3H8) combustion C3H8 + 5 O2 → 3 CO2 + 4 H2O 11. In the reaction of aluminum a ...
... d) Write a balanced equation for the decomposition of calcium carbonate into calcium oxide and carbon dioxide. CaCO3 → CaO + CO2 decomposition/ analysis e) Write a balanced equation for the complete combustion of propane (C3H8) combustion C3H8 + 5 O2 → 3 CO2 + 4 H2O 11. In the reaction of aluminum a ...
Chemical Equations Balancing Chemical Equations Try One…
... 1. start at the formula with the highest subscript values 2. put a “1” in front, if that doesn’t work try a “2”, etc. 3. go back and forth adding coefficients, until it’s ...
... 1. start at the formula with the highest subscript values 2. put a “1” in front, if that doesn’t work try a “2”, etc. 3. go back and forth adding coefficients, until it’s ...
Electrolysis of water
Electrolysis of water is the decomposition of water (H2O) into oxygen (O2) and hydrogen gas (H2) due to an electric current being passed through the water.This technique can be used to make hydrogen fuel (hydrogen gas) and breathable oxygen; though currently most industrial methods make hydrogen fuel from natural gas instead.