* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Electrochemistry
Geochemistry wikipedia , lookup
Electron configuration wikipedia , lookup
Cathodic protection wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Metallic bonding wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Transition state theory wikipedia , lookup
Double layer forces wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Theory of solar cells wikipedia , lookup
Artificial photosynthesis wikipedia , lookup
Stoichiometry wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Atomic theory wikipedia , lookup
Click chemistry wikipedia , lookup
Chemical reaction wikipedia , lookup
Water splitting wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Oxidation state wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Photoredox catalysis wikipedia , lookup
Gaseous detection device wikipedia , lookup
History of electrochemistry wikipedia , lookup
Electrolysis of water wikipedia , lookup
Metalloprotein wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
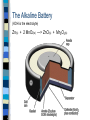
Electrochemistry Electrochemistry Electrochemistry involves either… – generating electricity by harnessing a spontaneous chemical reaction (one with K > 1) OR – using electricity to force a chemical reaction to occur (one that is non-spontaneous, K<1) Understanding electrochemistry requires a basic understanding of the processes called “oxidation” and “reduction”. Oxidation and Reduction Oxidation is… – the loss of electrons – an increase in oxidation state – the addition of oxygen – the loss of hydrogen Reduction is… – the gain of electrons – a decrease in oxidation state – the loss of oxygen – the addition of hydrogen 2 Mg + O2 2 MgO MgO + H2 Mg + H2O notice the magnesium is losing electrons notice the Mg2+ in MgO is gaining electrons Oxidation States Oxidation states are numbers assigned to atoms that reflect the net charge an atom would have if the electrons in the chemical bonds involving that atom were assigned to the more electronegative atoms. Oxidation states can be thought of as “imaginary” charges. They are assigned according to the following set of rules: Assigning Oxidation States Each atom in a pure element has an oxidation state of “0”. e.g. F2 K N2 Al3+ Fe2+ The oxidation state of hydrogen in a compound (e.g. CH3OH) is almost always “+1”. The sum of the oxidation states in a neutral compound must equal “0”. The sum of the oxidation states in a complex ion must equal the charge on the complex ion. Fe For mono-atomic ions, the oxidation state is equal to the charge on the ion. e.g. F- O3 O2- The oxidation state of oxygen in a compound (e.g. CH3OH) is almost always “-2”. Examples - assigning oxidation numbers Assign oxidation states to all elements: H2 K + Cr2O72ClO3 SO42- SO3 - - NH3 MnO4 CH3OH PO43- HSO3 - Cu Identifying Redox Reactions Oxidation and reduction always occur together in a chemical reaction. For this reason, these reactions are called “redox” reactions. Although there are different ways of identifying a redox reaction, the best is to look for a change in oxidation state: 2 Fe3+ + 2 I- 2 Fe2+ + I2 2 H2O 2 H2 + O2 2 AgNO3 + Cu 2 Ag + Cu(NO3)2 HCl + AgNO3 AgCl + HNO3 More Definitions Oxidizing Agent – the substance in a chemical reaction which causes another species to be oxidized. – the oxidizing agent always gets reduced in the reaction. Reducing Agent – the substance in a chemical reaction that causes another species to be reduced. – the reducing agent always gets oxidized! Examples - labeling redox reactions In each reaction, look for changes in oxidation state. If changes occur, identify the substance being reduced, and the substance being oxidized. Identify the oxidizing agent and the reducing agent. 5 Fe2+ + MnO4- + 8 H+ 5 Fe3+ + Mn2+ + 4 H2O H2 + CuO Cu + H2O Zn + 2 HCl ZnCl2 + H2 Balancing Redox Equations Redox reactions are often quite complicated and difficult to balance. For this reason, you’ll learn a step-by-step method for balancing these types of reactions, when they occur in acidic or in basic solutions. The procedure is called the “Half-Reactions Method” of balancing redox equations. It starts by identifying the substances being reduced and oxidized in the reaction, and then following the steps below: Balancing Redox Equations Half-Reactions Method First split the original equation into two half-reactions, one “reduction” and the other “oxidation”. In each half-reaction, follow these steps: Balance all elements except “H” and “O”. Balance the “O’s” by adding water, H2O. Balance the “H’s” by adding hydrogen ions, H+. Balance the electric charge by adding electrons, e-. Multiply the two equations by appropriate coefficients to make the # of electrons in the equations equal. Re-combine the two equations, canceling if needed. Example - Balancing with Half-Reactions Fe2+ + Cr2O72- Fe3+ + Cr3+ Fe2+ Fe3+ Cr2O72- Cr3+ Fe2+ Fe3+ + e- Cr2O72- 2 Cr3+ 6 Fe2+ Fe3+ + 6 e- Cr2O72- 2 Cr3+ + 7 H2O Cr2O72- + 14 H+ 2 Cr3+ + 7 H2O Cr2O72- + 14 H+ + 6 e- 2 Cr3+ + 7 H2O Cr2O72- + 6 Fe2+ + 14 H+ 2 Cr3+ + 6 Fe3+ + 7 H2O What if the solution was basic? Notice that the method has assumed the solution was acidic - we added H+ to balance the equation. The [H+] in a basic solution is very small. The [OH-] is much greater. For this reason, we will add enough OH- ions to both sides of the equation to neutralize the H+ in the overall reaction. The hydrogen and hydroxide ions will combine to make water, and you may have to do some canceling before you’re done. Cr2O72- + 6 Fe2+ + 7 H2O 2 Cr3+ + 6 Fe3+ + 14 OHWhy is this reaction unlikely in basic solution? Balancing Redox Equations Practice Balance in acidic solution: H2C2O4 + MnO4- Mn2+ + CO2 5 H2C2O4 + 2 MnO4- +16 H+ 2 Mn2+ + 10 CO2 + 8 H2O Balance in basic solution: CN- + MnO4- CNO- + MnO2 3 CN- + 2 MnO4- + H2O 3 CNO- + 2 MnO2 + 2 OH- Redox Reactions - What’s Happening? Zinc is added to a blue solution of copper(II) sulfate Zn (s) + CuSO4 (aq) ZnSO4 (aq) + Cu (s) The blue colour disappears…the zinc metal “dissolves”, and solid copper metal precipitates on the zinc strip The zinc is oxidized (loses electrons) The copper ions are reduced (gain Zn (s) + Cu2+ (aq) Zn2+ (aq) + Cu (s) electrons) Copper ions (Cu2+) collide with the zinc metal surface A zinc atom (Zn) gives up two of its electrons to the copper ion The result is a neutral atom of Cu deposited on the zinc strip, and a Zn2+ ion released into the solution Harnessing the Electricity During the spontaneous redox reaction, electrons flow from the zinc atoms to the copper ions. Zn Zn2+ + 2 eCu2+ + 2 e- Cu (oxidation) (reduction) Electricity can be thought of as the “flow of electric charge”. How can we make use of the flow of electrons from the zinc atoms to the copper ions?? Answer: SEPARATE the copper ions from the zinc atoms. This will force the electrons from the zinc atoms to travel through an external path to reach the copper ions. Electrochemical Cell – a device that uses a spontaneous redox reaction to produce electricity Anode – the electrode where oxidation occurs Cathode – the electrode where reduction occurs Salt Bridge – connects two “half-cells” to complete the electric circuit. – for example, a U-tube filled with salt solution Electrochemical Cells Definitions Electrochemical Cells Cell Potential Water flows spontaneously over a waterfall because of a difference in potential energy between the top of the falls and the stream below. In a similar fashion, electrons flow from the anode of a voltaic cell to the cathode because of a difference in potential energy. The potential energy of electrons is higher in the anode than in the cathode, and they spontaneously flow through an external circuit from the anode to the cathode. The potential difference between the two electrodes of an electrochemical cell provides the driving force that pushes electrons through the external circuit. We call this potential difference, denoted Ecell, the cell potential. Because Ecell is measured in volts, we often refer to it as the cell voltage. For any cell reaction that proceeds spontaneously, such as that in a voltaic cell, the cell voltage will be positive. Standard Hydrogen Electrode (SHE) It is impossible to measure the potential of a single electrode. Using a voltmeter, we can measure the difference in potential between two electrodes. Chemists arbitrarily assigned a potential of 0 V for the SHE. By measuring the difference in potential between the SHE and other electrodes, potentials can then be assigned to other electrodes. 2 H+ + 2 e- H2 E° = 0 V When a zinc electrode is connected to the SHE as shown above, the voltmeter reads: E°cell = + 0.76 V The positive sign means electrons are flowing from zinc to the hydrogen cell. The zinc is being oxidized. Since E°SHE= 0 V, we can conclude that E°Zn = + 0.76 V . This is the “oxidation potential” for the reaction: Zn Zn2+ + 2 e- Table of Standard Reduction Potentials Calculating a Cell Potential Step 1: Which electrode is more likely to be reduced? This is the cathode. Step 2: Write half-reactions each half-cell - one reduction and one oxidation reaction. Include the values for E°red and E°ox from the table of standard reduction potentials. Remember: E°red = -E°ox Step 3: Calculate the cell potential: E°cell = E°red + E°ox Step 1: Since Cu has a higher reduction potential than Zn, copper forms the cathode. We could also argue that Zn has a higher oxidation potential…and forms the anode. Step 2: Cathode: Cu2+ + 2e- Cu E°red = +0.34 V Anode: Zn Zn2+ + 2 eE°ox = +0.76 V Cu Step 3: The overall reaction is: Zn + Cu2+ Zn2+ + Cu E°cell = E°red + E°ox = 0.34 + 0.76 = 1.10 V Zn Line Notation An electrochemical cell needs to be described in a more convenient way than drawing a diagram! We use “line notation” to describe a cell. The zinccopper standard cell is described: Zn | Zn2+ (1.0 M) || Cu2+ (1.0 M) | Cu – – – – The ANODE is described before the CATHODE. Concentrations of ions are indicated in brackets. A vertical line represents a phase boundary. A double vertical line represents the salt bridge. Line Notation - an example Calculate the cell potential for the cell described below: Mg | Mg2+ (1.0 M) || Ag+ (1.0 M) | Ag Anode: Mg Mg2+ + 2 e- E° = 2.37 V (oxidation) Cathode: Ag+ + e- Ag E° = 0.80 V (reduction) Overall: Mg + 2 Ag+ Mg2+ + 2 Ag E° = 3.17 V Review: What’s the oxidizing agent? the reducing agent? What’s E° for the reverse reaction? The Nernst Equation So far, the cells we have considered have operated under standard conditions - 1.0 M solutions and 1.0 atm pressures at 25°C. Cell potential is dependent on concentration and on temperature, described by the Nernst Equation: RT Ecell E ln Qcell nF where “E°“ is the standard cell potential “R” is the Ideal Gas Constant, 8.314 “T” is the temperature of the cell “n” is the number of moles of electrons transferred “F” is the Faraday, the charge on one mole of electrons, 96 500 C/mol “Q” is the reaction quotient (remember your equilibrium unit!) Using the Nernst Equation Calculate Ecell for the cell described below at 25°C Mg | Mg2+ (0.0050 M) || Ag+ (2.0 M) | Ag – According to the balanced equation, Q [Mg 2 ] [ Ag ]2 – We have already seen that E°cell = 3.17 V – According to our balanced equation, n = 2 mol e– Substituting into the Nernst Equation: (0.0050 M) (8.314)(298 K) Ecell (3.17 V ) ln 2 (2 mol)(96500 C/mol) (2.0 M) Thus, Ecell = 3.26 V Calculating Equilibrium Constants When an electrochemical cell operates, the concentrations of the ions change until Q = K . When the cell reaches equilibrium, Ecell = 0. Combining these facts with the Nernst equation gives: Ecell 0 E RT ln K nF Rearranging, we can derive an equation to calculate the equilibrium constant for a redox reaction: RT E ln K nF Calculating K for a Redox Reaction Calculate K for the reaction that occurs when Mg is added to a solution of AgNO3. The net reaction is: Mg + 2 Ag+ Mg2+ + 2 Ag E RT ln K nF (8.314)(298 K) 3.17 V ln K (2 mol)(96500 C/mol) 247 = ln (K) so K = e247 = HUGE Corrosion Oxidation of metals with oxygen to form a metal oxide In some cases a thin layer of oxide coats the metal surface, preventing further oxidation (Al2O3) When iron corrodes, the process is called “rusting”. The iron oxide (Fe2O3) flakes off, exposing more metal to corrosion. Galvanized Iron - Preventing Corrosion Iron can be protected from corrosion by coating it with zinc metal - a metal that will oxidize first, instead of the iron Note that E°oxidation of zinc is greater than that of iron. Cathodic Protection - Preventing Corrosion To protect underground pipelines, a sacrificial anode is added. The water pipe is turned into the cathode and an active metal is used as the sacrificial anode. Magnesium is used as the sacrificial anode, since it is more easily oxidized than the iron water pipe. Dry-Cell Batteries Zn + 2 MnO2 + 2 NH4+ Zn+2 + Mn2O3 + 2 NH3 + H2O The Alkaline Battery (KOH is the electrolyte) Zn(s) + 2 MnO2(s) ---> ZnO(s) + Mn2O3(s) Lead Storage Battery (Car Battery) Anode: Pb (s) + HSO4- (aq) PbSO4 (s) + H+ (aq) + 2 e- Eo = 0.296 V Cathode: PbO2 (s) + 3 H+ (aq) + HSO4- (aq) + 2 e- PbSO4(s) + 2 H2O () Eo = 1.628 V Overall: Pb (s) + PbO2 (s) + 3 H+ (aq) + 2HSO4 2 PbSO4(s) + 2 H2O () Eo = 1.924 V A Picture of a Car Battery A 12-V battery has six cells connected in series. Each cell generates 2 V. Fuel Cells Anode: 2 H2 (g) + 4 OH- (aq) 2 H2O () + 4 eCathode: O2 (g) + 2 H2O () + 4 e- 4 OH- (aq) Overall: 2 H2 (g) + O2 (g) 2 H2O () Electrolysis of Molten Sodium Chloride Anode: Cathode: Overall: Molten sodium metal is formed at the cathode. Chlorine gas is formed at the anode. 2 Cl- () Cl2 (g) + 2 e2 Na+ () + 2 e- Na() 2 Cl- () 2 Na+ () Cl2 (g) + Na() Electrolysis of Water Anode: 2 H2O O2 + 4 H+ + 2 eCathode: 4 H2O + 4 e- 2 H2 + 4OH- H 2O Na2SO4 Overall: H2 O2 6 H2O O2 + 2 H2 + 4 OH- + 4 H+ Since 4 OH- + 4 H+ 4 H2O Net: 2 H2O O2 + 2 H2 cathode anode Electrolysis of Aqueous Sodium Bromide Two possible reactions at the cathode: 2 H2O + 2 e- H2 +2OHE° = -0.83 V Na+ + e- Na E° = -2.71 V Two possible reactions at the anode: 2 Br- Br2 + 2 eE° = -1.09 V 2 H2O O2 + 4 H+ + 4 eE° = -1.23 V Overall reaction: 2 Br- + 2 H2O Br2 + H2 + 2 OHEocell = Eored + Eoox = -0.83 + (-1.09) Eocell = -1.92 V Quantitative Electrolysis Q =It Charge = Current x time (Coulombs) (Amperes) (seconds) 1 F = 96 500 C/mol e- What mass of aluminum can be produced in 8.00 min by passing a constant current of 100 A through a molten mixture of aluminum oxide, Al2O3? Al3+ + 3 e- Al (s) 60 s 100 C 1mol e 1mol Al 26.98 g Al 8.00 min 1min 1 sec 96 500 C 3 mol e 1mol Al = 4.47 g of Aluminum will be produced