Chapter 21 Nonmetallic Elements and Their Compounds
... electrolysis of carbon tetrachloride. oxidation of chloride ion with F2(g). electrolysis of NaCl(aq). oxidation of chloride ion with Br2(aq). electrolysis of AlCl3(aq). ...
... electrolysis of carbon tetrachloride. oxidation of chloride ion with F2(g). electrolysis of NaCl(aq). oxidation of chloride ion with Br2(aq). electrolysis of AlCl3(aq). ...
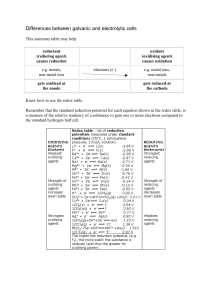
Differences between galvanic and electrolytic cells
... (oxidising agent) causes oxidation electrons (e–) ...
... (oxidising agent) causes oxidation electrons (e–) ...
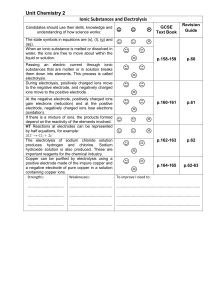
Unit_Chemistry_2_Ionic_Substances_and_Electrolysis
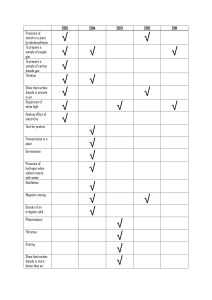
... Ionic Substances and Electrolysis Candidates should use their skills, knowledge and understanding of how science works: ...
... Ionic Substances and Electrolysis Candidates should use their skills, knowledge and understanding of how science works: ...
Electrolysis of water
Electrolysis of water is the decomposition of water (H2O) into oxygen (O2) and hydrogen gas (H2) due to an electric current being passed through the water.This technique can be used to make hydrogen fuel (hydrogen gas) and breathable oxygen; though currently most industrial methods make hydrogen fuel from natural gas instead.