blood - SCH4U1-02-2010
... - The ideal pH level for blood is 7.35-7.45 - The higher the pH of a solution, the more electrical resistance that solution holds. - If pH level is either alkaline or acid, you will become ill, perhaps even die. 6. Electrolyte -Dietary Electrolytes and Blood Pressure. Electrolyte is a "medical/scien ...
... - The ideal pH level for blood is 7.35-7.45 - The higher the pH of a solution, the more electrical resistance that solution holds. - If pH level is either alkaline or acid, you will become ill, perhaps even die. 6. Electrolyte -Dietary Electrolytes and Blood Pressure. Electrolyte is a "medical/scien ...
word - University of Guelph
... chemicals and organic solvents in glas apparatus, often under reflux conditions with continous water cooling and under inert gas atmosphere or vacuum on a scale of 10 to 1000 mL total volume. Typical maximum heating temperatures are below 200 °C. Occasionally reactions will be carried out in 50 to 5 ...
... chemicals and organic solvents in glas apparatus, often under reflux conditions with continous water cooling and under inert gas atmosphere or vacuum on a scale of 10 to 1000 mL total volume. Typical maximum heating temperatures are below 200 °C. Occasionally reactions will be carried out in 50 to 5 ...
Ch 2-1 Properties of Matter
... 72) The wax appears to disappear because the products of the reaction—carbon dioxide and water vapor—are colorless. 79) a) yes; because the graph is a straight line, the proportion of iron to oxygen is a constant, which is true for a compound. b) no; a point for the values given wouldn’t fall on the ...
... 72) The wax appears to disappear because the products of the reaction—carbon dioxide and water vapor—are colorless. 79) a) yes; because the graph is a straight line, the proportion of iron to oxygen is a constant, which is true for a compound. b) no; a point for the values given wouldn’t fall on the ...
Exam practice answers 5
... to give the overall equation: 2MnO4–(aq) + 16H+(aq) + 10I–(aq) 2Mn2+ + 8H2O(l) + 5I2(aq) 4 (a) Hydrogen and oxygen are supplied to the fuel cell. If an acidic catalyst is used: At the positive electrode the fuel (hydrogen gas) is converted to hydrogen ions: H2(g) 2H+(aq) + 2e– At the negat ...
... to give the overall equation: 2MnO4–(aq) + 16H+(aq) + 10I–(aq) 2Mn2+ + 8H2O(l) + 5I2(aq) 4 (a) Hydrogen and oxygen are supplied to the fuel cell. If an acidic catalyst is used: At the positive electrode the fuel (hydrogen gas) is converted to hydrogen ions: H2(g) 2H+(aq) + 2e– At the negat ...
Intermolecular Forces
... neighbouring molecules as a result of a temporary imbalance in the position of the atoms’ electrons -forms between all molecules, polar and nonpolar - the side of the atoms with more electrons develops a temporary negative charge, and the side with fewer electrons develops a temporary positive charg ...
... neighbouring molecules as a result of a temporary imbalance in the position of the atoms’ electrons -forms between all molecules, polar and nonpolar - the side of the atoms with more electrons develops a temporary negative charge, and the side with fewer electrons develops a temporary positive charg ...
C07 Introduction to Chemical Reactions
... 2. Skeletal Equations A skeletal equation is one that takes the chemical names of a compound and converts them to their chemical formula. The advantage to the skeletal equation is that it gives you a sense of the number of atoms involved in a reaction. Examples word equation hydrogen (g) ...
... 2. Skeletal Equations A skeletal equation is one that takes the chemical names of a compound and converts them to their chemical formula. The advantage to the skeletal equation is that it gives you a sense of the number of atoms involved in a reaction. Examples word equation hydrogen (g) ...
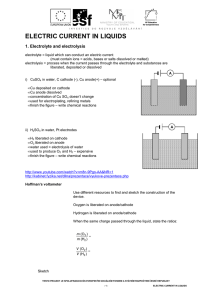
ELECTRIC CURRENT IN LIQUIDS
... 1. Electrolyte and electrolysis electrolyte = liquid which can conduct an electric current (must contain ions = acids, bases or salts dissolved or melted) electrolysis = process when the current passes through the electrolyte and substances are liberated, deposited or dissolved A i) CuSO4 in water, ...
... 1. Electrolyte and electrolysis electrolyte = liquid which can conduct an electric current (must contain ions = acids, bases or salts dissolved or melted) electrolysis = process when the current passes through the electrolyte and substances are liberated, deposited or dissolved A i) CuSO4 in water, ...
Electrolysis, the Faraday, and Avogadro`s Number
... The passage of an electric current through a solution is accompanied by chemical reactions at the electrodes. Oxidation occurs at the anode; reduction occurs at the cathode. The amount of reaction that occurs at the electrodes is directly proportional to the number of electrons transferred. Thus, a ...
... The passage of an electric current through a solution is accompanied by chemical reactions at the electrodes. Oxidation occurs at the anode; reduction occurs at the cathode. The amount of reaction that occurs at the electrodes is directly proportional to the number of electrons transferred. Thus, a ...
Hydrogen as Energy Carrier Part 1 Powerpoint
... UCLA Science and Engineering of the Environment GK-12 ...
... UCLA Science and Engineering of the Environment GK-12 ...
Answers to Review Questions
... absorb, and also playing a part in movement of water in a plant. The high specific heat of water is also due to hydrogen bonding, and is important in modulating the body temperature of living things, as they are composed primarily of water. Hydrogen bonding also results in the characteristic of ice ...
... absorb, and also playing a part in movement of water in a plant. The high specific heat of water is also due to hydrogen bonding, and is important in modulating the body temperature of living things, as they are composed primarily of water. Hydrogen bonding also results in the characteristic of ice ...
High-energy Hydrogen III Teacher Page Electrolysis
... When electricity is applied, bubbles of oxygen gas (O2) form at the anode, and bubbles of hydrogen gas (H2) form at the cathode. The bubbles are easily seen. Twice as much hydrogen gas is produced as oxygen gas. At the anode, water is oxidized: 2H2O 6 O2 + 4H+ + 4eAt the cathode, water is reduced: 4 ...
... When electricity is applied, bubbles of oxygen gas (O2) form at the anode, and bubbles of hydrogen gas (H2) form at the cathode. The bubbles are easily seen. Twice as much hydrogen gas is produced as oxygen gas. At the anode, water is oxidized: 2H2O 6 O2 + 4H+ + 4eAt the cathode, water is reduced: 4 ...
CHEMISTRY
... Reactants: compounds rearranged (left side) Products: compounds created (right side) ...
... Reactants: compounds rearranged (left side) Products: compounds created (right side) ...
Intro Biochemistry/Ecology
... The main types of chemical bonds are covalent bonds and ionic bonds. Section 2-2: Properties of Water A water molecule is polar, because there is an uneven distribution of electrons between the oxygen and hydrogen atoms. Acidic solutions contain higher concentrations of H+ ions than pure water and h ...
... The main types of chemical bonds are covalent bonds and ionic bonds. Section 2-2: Properties of Water A water molecule is polar, because there is an uneven distribution of electrons between the oxygen and hydrogen atoms. Acidic solutions contain higher concentrations of H+ ions than pure water and h ...
Answers for Review Questions Exam 3
... cell reaction reaches equilibrium, the battery is dead. Ex. Dry cell battery, Mercury, Lithium batteries, Alkaline Batteries. Problems: Can’t be reused, Dry battery- produces gaseous products, poor shelf life. Mercury Battery – poisonous. Secondary Battery – Uses a redox reaction, which is recharged ...
... cell reaction reaches equilibrium, the battery is dead. Ex. Dry cell battery, Mercury, Lithium batteries, Alkaline Batteries. Problems: Can’t be reused, Dry battery- produces gaseous products, poor shelf life. Mercury Battery – poisonous. Secondary Battery – Uses a redox reaction, which is recharged ...
Answers for Review Questions Exam 3
... cell reaction reaches equilibrium, the battery is dead. Ex. Dry cell battery, Mercury, Lithium batteries, Alkaline Batteries. Problems: Can’t be reused, Dry battery- produces gaseous products, poor shelf life. Mercury Battery – poisonous. Secondary Battery – Uses a redox reaction, which is recharged ...
... cell reaction reaches equilibrium, the battery is dead. Ex. Dry cell battery, Mercury, Lithium batteries, Alkaline Batteries. Problems: Can’t be reused, Dry battery- produces gaseous products, poor shelf life. Mercury Battery – poisonous. Secondary Battery – Uses a redox reaction, which is recharged ...
Electrolysis of water
Electrolysis of water is the decomposition of water (H2O) into oxygen (O2) and hydrogen gas (H2) due to an electric current being passed through the water.This technique can be used to make hydrogen fuel (hydrogen gas) and breathable oxygen; though currently most industrial methods make hydrogen fuel from natural gas instead.