Name ……………………………..………...… …….. Index No
... to obtain hydrogen and oxygen gases. Study it and answer the questions that follow. Hydrogen gas ...
... to obtain hydrogen and oxygen gases. Study it and answer the questions that follow. Hydrogen gas ...
Unit 8-10 Review Answers
... 3. All of the following statements about liquids and gases are true except . (a) Molecules in a liquid are much more closely packed than molecules in a gas. (b) Molecules in a liquid can vibrate and rotate, but they cannot move about freely as molecules in a gas. (c) Liquids are much more difficult ...
... 3. All of the following statements about liquids and gases are true except . (a) Molecules in a liquid are much more closely packed than molecules in a gas. (b) Molecules in a liquid can vibrate and rotate, but they cannot move about freely as molecules in a gas. (c) Liquids are much more difficult ...
8th_Science_section_II_Objective_four_test
... C. Matter is created when a chemical reaction takes place D. molecules combine to form elements ...
... C. Matter is created when a chemical reaction takes place D. molecules combine to form elements ...
File
... • Diatomic molecules contain two atoms. • Seven elements exist as diatomic molecules at room temperature. ▫ H2, N2, O2, F2, Cl2, Br2, I2 ...
... • Diatomic molecules contain two atoms. • Seven elements exist as diatomic molecules at room temperature. ▫ H2, N2, O2, F2, Cl2, Br2, I2 ...
sch3u unit 1 test: matter
... 25. __ Gold is a highly reactive metal. 26. ___ Barium hydroxide produced in a double displacement reaction will precipitate out. 27. ___ Hydrogen is in the activity series because it classifies as a metal. SECTION B: THINKING/INQUIRY (30 marks) 1. Draw the following Lewis symbols/Lewis structures ( ...
... 25. __ Gold is a highly reactive metal. 26. ___ Barium hydroxide produced in a double displacement reaction will precipitate out. 27. ___ Hydrogen is in the activity series because it classifies as a metal. SECTION B: THINKING/INQUIRY (30 marks) 1. Draw the following Lewis symbols/Lewis structures ( ...
Atomic Number and Mass Number
... How are the atomic number and the number of protons related to each other? Atomic number = number of protons How do the number of protons, number of neutrons, and the mass number relate to each other? # of protons + # of neutrons = mass # What is the one thing that determines the identity of an atom ...
... How are the atomic number and the number of protons related to each other? Atomic number = number of protons How do the number of protons, number of neutrons, and the mass number relate to each other? # of protons + # of neutrons = mass # What is the one thing that determines the identity of an atom ...
Sodium hydroxide
... • Removing O2 from a substance is called reduction. The copper oxide is reduced to copper. • Copper is purified by electrolysis. Electricity is passed through solutions containing copper compounds, such as copper(II) sulphate. Pure copper forms on the negative electrode. The animation shows how this ...
... • Removing O2 from a substance is called reduction. The copper oxide is reduced to copper. • Copper is purified by electrolysis. Electricity is passed through solutions containing copper compounds, such as copper(II) sulphate. Pure copper forms on the negative electrode. The animation shows how this ...
ExamView - Untitled.tst
... b. stays the same. d. varies. ____ 11. A state of matter that is not a fluid is a. water. c. liquid. b. gas. d. solid. ____ 12. Dalton’s atomic theory was accepted because a. there was evidence to support it. b. Democritus said that it was correct. c. Dalton invented the electron microscope. d. Dalt ...
... b. stays the same. d. varies. ____ 11. A state of matter that is not a fluid is a. water. c. liquid. b. gas. d. solid. ____ 12. Dalton’s atomic theory was accepted because a. there was evidence to support it. b. Democritus said that it was correct. c. Dalton invented the electron microscope. d. Dalt ...
Elementary my dear Watson review
... Remember the vinegar and baking soda lab we did: the mass of the reactors (baking soda and vinegar) was equal to the mass of the product (fizz!) only when we placed a balloon on top of the Erlenmeyer Flask to catch the gas (CO2) that was produced. Otherwise, the mass after the reaction would have de ...
... Remember the vinegar and baking soda lab we did: the mass of the reactors (baking soda and vinegar) was equal to the mass of the product (fizz!) only when we placed a balloon on top of the Erlenmeyer Flask to catch the gas (CO2) that was produced. Otherwise, the mass after the reaction would have de ...
Solutions - Seattle Central
... Benedict's solution is a chemical indicator for simple sugars such as glucose: C6H12O6. Unlike some other indicators, Benedict’s solution does not work at room temperature - it must be heated first ...
... Benedict's solution is a chemical indicator for simple sugars such as glucose: C6H12O6. Unlike some other indicators, Benedict’s solution does not work at room temperature - it must be heated first ...
Chemistry Notes
... Separate the water in salt water from the salts Boil off the water and salts will remain Separate a mixture of gases Cool them – they will condense at different temperatures ...
... Separate the water in salt water from the salts Boil off the water and salts will remain Separate a mixture of gases Cool them – they will condense at different temperatures ...
Test Objectives for Unit 11: Oxidation/Reduction
... In an electrochemical (or galvanic or voltaic) cell, a spontaneous chemical reaction is used to produce electricity. (spontaneous reaction → electricity) This is an exothermic process. What type of energy is produced? Identify the components of an electrochemical cell and describe their function. (h ...
... In an electrochemical (or galvanic or voltaic) cell, a spontaneous chemical reaction is used to produce electricity. (spontaneous reaction → electricity) This is an exothermic process. What type of energy is produced? Identify the components of an electrochemical cell and describe their function. (h ...
Catalyst Activity (in your notebook)
... • the speed of the reaction – depends on chemical kinetics – can be very slow, almost unnoticeably ...
... • the speed of the reaction – depends on chemical kinetics – can be very slow, almost unnoticeably ...
Acid Spill - Rosshall Academy
... C. An acid that has been neutralised by an alkali D. An acid with the hydrogen ions replaced by ammonium ions 17 The salts formed by nitric acid are called A. chlorides B. ethanoates C. nitrates D. sulphates 18 In the reaction between nitric acid and zinc oxide, one of the products is zinc nitrate, ...
... C. An acid that has been neutralised by an alkali D. An acid with the hydrogen ions replaced by ammonium ions 17 The salts formed by nitric acid are called A. chlorides B. ethanoates C. nitrates D. sulphates 18 In the reaction between nitric acid and zinc oxide, one of the products is zinc nitrate, ...
Chemistry Wksht 26
... 3. What is the difference between a strong electrolyte and a weak electrolyte? 4. Give two examples each of a strong electrolyte and a weak electrolyte. 5. Why does water conduct electricity? 6. Write the equation for the self ionization of water. 7. What is the equilibrium constant expression for w ...
... 3. What is the difference between a strong electrolyte and a weak electrolyte? 4. Give two examples each of a strong electrolyte and a weak electrolyte. 5. Why does water conduct electricity? 6. Write the equation for the self ionization of water. 7. What is the equilibrium constant expression for w ...
GS130: Physical World Worksheet for Exam 3
... 2. a) Write down the balanced equation for the oxidation of hydrogen to form water. b) When this reaction happens, 13.67 kJ per gram of water is given off. c) Is this an exothermic or an endothermic reaction? d) How much energy do you need for the electrolytic decomposition of 1 liter of water into ...
... 2. a) Write down the balanced equation for the oxidation of hydrogen to form water. b) When this reaction happens, 13.67 kJ per gram of water is given off. c) Is this an exothermic or an endothermic reaction? d) How much energy do you need for the electrolytic decomposition of 1 liter of water into ...
ch_24_poss_elmo
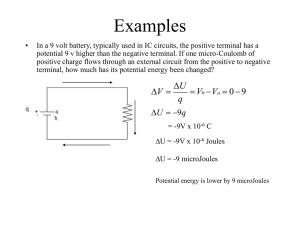
... Examples A proton is placed in an electric field of E=105 V/m and released. After going 10 cm, what is its speed? Use conservation of energy. a ...
... Examples A proton is placed in an electric field of E=105 V/m and released. After going 10 cm, what is its speed? Use conservation of energy. a ...
Electrolysis of water
Electrolysis of water is the decomposition of water (H2O) into oxygen (O2) and hydrogen gas (H2) due to an electric current being passed through the water.This technique can be used to make hydrogen fuel (hydrogen gas) and breathable oxygen; though currently most industrial methods make hydrogen fuel from natural gas instead.