C1a - Mr Corfe
... Chloride – bleaches litmas paper When above is reacted with water Element + water → Element hydroxide + hydrogen REACTIVITY SERIES Most reactive least reactive caesium Cs rubidium Rb potassium K sodium Na lithium Li calcium Ca magnesium Mg aluminium Al zinc Zn iron Fe Gold Au s ...
... Chloride – bleaches litmas paper When above is reacted with water Element + water → Element hydroxide + hydrogen REACTIVITY SERIES Most reactive least reactive caesium Cs rubidium Rb potassium K sodium Na lithium Li calcium Ca magnesium Mg aluminium Al zinc Zn iron Fe Gold Au s ...
Year 8 Science Assessment Point 2
... 1. Reversible reaction: A reaction that can go backwards and the products be converted back into the reactants 2. Exothermic reaction: A reaction that releases heat so feels hot 3. Endothermic reaction: A reaction that takes in heat from its surroundings to feels cold ...
... 1. Reversible reaction: A reaction that can go backwards and the products be converted back into the reactants 2. Exothermic reaction: A reaction that releases heat so feels hot 3. Endothermic reaction: A reaction that takes in heat from its surroundings to feels cold ...
Name: Date: Block:______ GRADE 8 SCIENCE SOL QUESTIONS
... not pure c. they did not add the correct material to the common metal d. elements cannot be changed to other elements by physical or chemical means 20. Mercury is a liquid metal that is used in many thermometers. The mercury in the thermometer rises because it — a. is sucked upward by vacuum b. expa ...
... not pure c. they did not add the correct material to the common metal d. elements cannot be changed to other elements by physical or chemical means 20. Mercury is a liquid metal that is used in many thermometers. The mercury in the thermometer rises because it — a. is sucked upward by vacuum b. expa ...
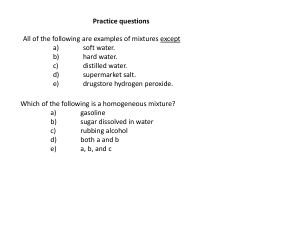
Practice questions
... a) neutrons. b) atomic number. c) nuclear charge. d) electron configuration. e) number of protons. ...
... a) neutrons. b) atomic number. c) nuclear charge. d) electron configuration. e) number of protons. ...
Answers to 2017 Chemistry Exam Review Compounds and
... 41. Water tends to dissolve other polar substances since its positive pole will be attracted to the negative pole of the other substance and vice versa. It tends not to dissolve nonpolar substances b/c there are no poles for water’s poles to be attracted to. 42. Ca(NO3)2 Ca2+ + 2 NO3143. A precipita ...
... 41. Water tends to dissolve other polar substances since its positive pole will be attracted to the negative pole of the other substance and vice versa. It tends not to dissolve nonpolar substances b/c there are no poles for water’s poles to be attracted to. 42. Ca(NO3)2 Ca2+ + 2 NO3143. A precipita ...
Document
... This claim is false because it — F violates the principle of constant composition G contradicts the law of conservation of matter H ignores the strength of the theory of strings J violates the rules of gravitational attraction ...
... This claim is false because it — F violates the principle of constant composition G contradicts the law of conservation of matter H ignores the strength of the theory of strings J violates the rules of gravitational attraction ...
IntroRedoxDCIAns
... appearance of an element. The other characteristic is the change in the number of oxygen atoms around the non-oxygen elements in the reactions. c. Explain the historic interpretation of oxidation and reduction in chemical reactions. Historically oxidation was defined as occurring when the number of ...
... appearance of an element. The other characteristic is the change in the number of oxygen atoms around the non-oxygen elements in the reactions. c. Explain the historic interpretation of oxidation and reduction in chemical reactions. Historically oxidation was defined as occurring when the number of ...
Introduction to Oxidation Reduction
... appearance of an element. The other characteristic is the change in the number of oxygen atoms around the non-oxygen elements in the reactions. c. Explain the historic interpretation of oxidation and reduction in chemical reactions. Historically oxidation was defined as occurring when the number of ...
... appearance of an element. The other characteristic is the change in the number of oxygen atoms around the non-oxygen elements in the reactions. c. Explain the historic interpretation of oxidation and reduction in chemical reactions. Historically oxidation was defined as occurring when the number of ...
Prelim Revision Paper 4
... The properties of fractions obtained from crude oil depend on the sizes of molecules in the fractions. Compared with a fraction containing small molecules, a fraction containing large molecules will A ...
... The properties of fractions obtained from crude oil depend on the sizes of molecules in the fractions. Compared with a fraction containing small molecules, a fraction containing large molecules will A ...
Chemistry 121 - Oregon State chemistry
... A marathon race covers a distance of 42.195 km. What is this distance in meters? In ...
... A marathon race covers a distance of 42.195 km. What is this distance in meters? In ...
activity series
... 3. A small delta, (∆), above the arrow shows that heat has been added. 4. Before beginning to balance an equation, check each formula to see that it is correct. NEVER change a formula during the balancing of an equation. 5. Balancing is done by placing coefficients in front of the formulas to insure ...
... 3. A small delta, (∆), above the arrow shows that heat has been added. 4. Before beginning to balance an equation, check each formula to see that it is correct. NEVER change a formula during the balancing of an equation. 5. Balancing is done by placing coefficients in front of the formulas to insure ...
CHEM 150
... 31. In the space provided, draw the Lewis dot structure of (remember to include lone pair electrons, if present, into your drawing): a. CH3NH2 (3 points) ...
... 31. In the space provided, draw the Lewis dot structure of (remember to include lone pair electrons, if present, into your drawing): a. CH3NH2 (3 points) ...
standard sample test
... III. Show your thinking process. Answer the question as concisely as possible in the space provided. (1 question, 20 points) 16. A teaspoon holds 2.00 mL of pure water at 27.0˚C. A student pours out the water at a rate of 1,000 molecules per second. Could the student pour out all the water while ho ...
... III. Show your thinking process. Answer the question as concisely as possible in the space provided. (1 question, 20 points) 16. A teaspoon holds 2.00 mL of pure water at 27.0˚C. A student pours out the water at a rate of 1,000 molecules per second. Could the student pour out all the water while ho ...
Introduction to Chemical Equations
... Matter is being rearranged, but NO mass is lost. If you were to collect all of the products and measure their mass, it would be equal to the original mass of the wood. ...
... Matter is being rearranged, but NO mass is lost. If you were to collect all of the products and measure their mass, it would be equal to the original mass of the wood. ...
C4C5C6
... Strong and Weak Acids • Strong Acids ionise completely in water. This means that the compound dissociates (e.g HCl H+ + Cl-). There is a higher concentration of H+ ions ready to react. • Weak Acids only partially ionise in water. It is a ...
... Strong and Weak Acids • Strong Acids ionise completely in water. This means that the compound dissociates (e.g HCl H+ + Cl-). There is a higher concentration of H+ ions ready to react. • Weak Acids only partially ionise in water. It is a ...
Exam #2
... The solution to the Schrodinger wave equation for the hydrogen atom does not provide a detailed description of the electron’s position but only the probability of finding the electron in a given region of space. The phenomenon of radioactivity was discovered by Becquerel who observed that uranium sa ...
... The solution to the Schrodinger wave equation for the hydrogen atom does not provide a detailed description of the electron’s position but only the probability of finding the electron in a given region of space. The phenomenon of radioactivity was discovered by Becquerel who observed that uranium sa ...
1) COMBINATION REACTION
... 1) COMBINATION REACTION (SYNTHESIS). HERE, TWO REACTANTS COMBINE TO GIVE A PRODUCT. A + B AB AN EXAMPLE WOULD BE MAGNESIUM METAL REACTING WITH OXYGEN TO FORM MgO, MAGNESIUM OXIDE. 2Mg + O2 2 MgO ...
... 1) COMBINATION REACTION (SYNTHESIS). HERE, TWO REACTANTS COMBINE TO GIVE A PRODUCT. A + B AB AN EXAMPLE WOULD BE MAGNESIUM METAL REACTING WITH OXYGEN TO FORM MgO, MAGNESIUM OXIDE. 2Mg + O2 2 MgO ...
FIREWORKS EMC summary notes
... are not easily reversed; they are irreversible. In a physical change no new substance is formed. Melting and evaporation are examples of physical changes. Physical changes are usually reversible. You can tell that a reaction has occurred if there is a colour change or when a gas is given off. Most c ...
... are not easily reversed; they are irreversible. In a physical change no new substance is formed. Melting and evaporation are examples of physical changes. Physical changes are usually reversible. You can tell that a reaction has occurred if there is a colour change or when a gas is given off. Most c ...
Chemistry Review - Woodlawn School Wiki
... 1) In my lab, I have an unknown solution in a beaker that could possibly have ions of silver, strontium or iron(III). I added rubidium iodide and nothing precipitated out. I added a solution of sodium hydroxide and received a precipitate. I finally added a solution potassium sulfate and a precipitat ...
... 1) In my lab, I have an unknown solution in a beaker that could possibly have ions of silver, strontium or iron(III). I added rubidium iodide and nothing precipitated out. I added a solution of sodium hydroxide and received a precipitate. I finally added a solution potassium sulfate and a precipitat ...
Electrolysis of water
Electrolysis of water is the decomposition of water (H2O) into oxygen (O2) and hydrogen gas (H2) due to an electric current being passed through the water.This technique can be used to make hydrogen fuel (hydrogen gas) and breathable oxygen; though currently most industrial methods make hydrogen fuel from natural gas instead.