Chemistry Review for End of year final honors
... 9.) Remember that all ΔH values for elements are not given since they are assumed to be zero. Gas Laws 1.) List the five gas laws and give their formulas 2.) What happens to the temperature of a gas when it is compressed? 3.) A gas occupies a volume of 0.2 L at 10. kPa. What volume will it occupy at ...
... 9.) Remember that all ΔH values for elements are not given since they are assumed to be zero. Gas Laws 1.) List the five gas laws and give their formulas 2.) What happens to the temperature of a gas when it is compressed? 3.) A gas occupies a volume of 0.2 L at 10. kPa. What volume will it occupy at ...
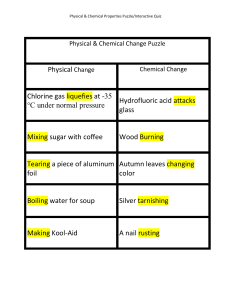
Physical Change Chlorine gas liquefies at
... Physical & Chemical Properties Puzzle/Interactive Quiz ...
... Physical & Chemical Properties Puzzle/Interactive Quiz ...
File
... (ice → water @ 0˚ C) Boiling Point : high or low (water → water vapour @100˚ C ) Density = Every substance has a unique density (g/ml) Solubility : soluble or insoluble. Capacity to dissolve in another substance Viscosity: high or low. The measure of a substance’s resistance to flow. (e.g. maple syr ...
... (ice → water @ 0˚ C) Boiling Point : high or low (water → water vapour @100˚ C ) Density = Every substance has a unique density (g/ml) Solubility : soluble or insoluble. Capacity to dissolve in another substance Viscosity: high or low. The measure of a substance’s resistance to flow. (e.g. maple syr ...
Final Exam Review – Free Response Section Name: 1. A sample of
... 7. 2.00 L of a gas at 25 oC and 1.05 atm is heated to 30 oC and the pressure is reduced to 0.550 atm. Calculate the new volume. ...
... 7. 2.00 L of a gas at 25 oC and 1.05 atm is heated to 30 oC and the pressure is reduced to 0.550 atm. Calculate the new volume. ...
Chapter 6: Chemistry in Biology
... Substances that release hydroxide ions ( OH ) when dissolved in water are called __________. pH and Buffers: The measure of concentration of H in a solution is called __________. ...
... Substances that release hydroxide ions ( OH ) when dissolved in water are called __________. pH and Buffers: The measure of concentration of H in a solution is called __________. ...
Chemistry of Life
... and positive sides of the water molecules. Water has a partial negative charge due to the extra unshared e- that Oxygen and a partial + charge near the hydrogen atoms ...
... and positive sides of the water molecules. Water has a partial negative charge due to the extra unshared e- that Oxygen and a partial + charge near the hydrogen atoms ...
Atmosphere Test Review Practice
... 3. What process is shown at point A? Is water a liquid, solid or gas during this process? 4. What process is shown at point B? Is water a liquid, solid or gas during this process? 5. What process is shown at point C? Is water a liquid, solid or gas during this process? ...
... 3. What process is shown at point A? Is water a liquid, solid or gas during this process? 4. What process is shown at point B? Is water a liquid, solid or gas during this process? 5. What process is shown at point C? Is water a liquid, solid or gas during this process? ...
Lecture 1.1 Some preliminary chemistry knowledge, ppt file
... is held in its orbital by its attraction to the proton in the nucleus. ...
... is held in its orbital by its attraction to the proton in the nucleus. ...
Chapter 12 Alcohols, Phenols, Ethers, Aldehydes, and Ketones
... These reactions illustrates two important principles. 1st, the more reduced a molecule, the more energy is released during oxidation on a molar basis. Methane is fully reduced and gives off more energy during combustion than methanol. 2nd, the number of oxygen molecules required to react with a fue ...
... These reactions illustrates two important principles. 1st, the more reduced a molecule, the more energy is released during oxidation on a molar basis. Methane is fully reduced and gives off more energy during combustion than methanol. 2nd, the number of oxygen molecules required to react with a fue ...
Topic 8.4 Acids and Bases The pH Scale
... Scale between 1 and 14 pH 7 is neutral Acids are from 0 to 6.99 ...
... Scale between 1 and 14 pH 7 is neutral Acids are from 0 to 6.99 ...
IN-SITU CHARACTERIZATION OF SPUTTERED PD THIN-FILMS UNDERGOING ELECTROLYSIS
... Figure 1. (a) Schematic for the resistance measurement. (b) A Pd/Ni electrode with electrical leads attached through indium contacts The immediate problem encountered in measuring resistance during electrolysis is the bypass (short-circuit) effect of electrolyte. For a wire experiment [2], this is n ...
... Figure 1. (a) Schematic for the resistance measurement. (b) A Pd/Ni electrode with electrical leads attached through indium contacts The immediate problem encountered in measuring resistance during electrolysis is the bypass (short-circuit) effect of electrolyte. For a wire experiment [2], this is n ...
Ivan Lomachenkov
... the ions of Na. The result is vr~ I/n, I- the current, n- the concentration of the ions. For the current I~ 0.1 A we have vr~ 10-7m/s. • We can also estimate the circular component of the velocity: v~ nvrB/, where - the viscosity of the solution. For inductance B~10-2 T and ~10-3kg/(m·s) the re ...
... the ions of Na. The result is vr~ I/n, I- the current, n- the concentration of the ions. For the current I~ 0.1 A we have vr~ 10-7m/s. • We can also estimate the circular component of the velocity: v~ nvrB/, where - the viscosity of the solution. For inductance B~10-2 T and ~10-3kg/(m·s) the re ...
Chemistry of Life - juan-roldan
... ◦ Example is hydrogen gas molecule ◦ Bond can be single, double, or triple ...
... ◦ Example is hydrogen gas molecule ◦ Bond can be single, double, or triple ...
1 - KCSE Online
... (c) Mg3N2(s) + 6H2O(l) →3Mg(OH)2(aq) + 2NH3(g) √ 1mk (d) (i) Passing gas X/NH3 through heated √ 1mk Copper (III) oxide (CuO) where ammonia is √ 1mk oxidize to nitrogen/(Y) (ii) 2NH3(g) + 3CuO (s) → N2(g) + 3Cu(s) + 3H2O(l) √ 1mk (e) Carbon (IV) oxide/CO2 √ 1mk (f) Any noble gases (He, Ne, Ar) √ 1mk ...
... (c) Mg3N2(s) + 6H2O(l) →3Mg(OH)2(aq) + 2NH3(g) √ 1mk (d) (i) Passing gas X/NH3 through heated √ 1mk Copper (III) oxide (CuO) where ammonia is √ 1mk oxidize to nitrogen/(Y) (ii) 2NH3(g) + 3CuO (s) → N2(g) + 3Cu(s) + 3H2O(l) √ 1mk (e) Carbon (IV) oxide/CO2 √ 1mk (f) Any noble gases (He, Ne, Ar) √ 1mk ...
On_the__optimization_of_electrolysis_Corotto_edit_2
... Many useful gases can be extracted through electrolysis of liquids. If one were to extract hydrogen from water for propulsion of spacecraft or earthly vehicles, it would be better, if not downright necessary, to do it in the optimum way. Based on the density of water and the ideal gas law, it can be ...
... Many useful gases can be extracted through electrolysis of liquids. If one were to extract hydrogen from water for propulsion of spacecraft or earthly vehicles, it would be better, if not downright necessary, to do it in the optimum way. Based on the density of water and the ideal gas law, it can be ...
Section 1 Atoms, Elements, and Compounds
... Balancing o The number of atoms must be the same on both sides of an equation. ...
... Balancing o The number of atoms must be the same on both sides of an equation. ...
Chemical Changes in Matter Worksheet
... 1. Two atoms of solid lithium react with two molecules of liquid water to produce two units of aqueous lithium hydroxide and one molecule of hydrogen gas. ...
... 1. Two atoms of solid lithium react with two molecules of liquid water to produce two units of aqueous lithium hydroxide and one molecule of hydrogen gas. ...
Ch. 20 study questions
... cathode half-cell had decreased to .50 M a. Calculate the initial cell potential b. Calculate the cell potential at time, t. c. Calculate the total charge provided by the cell. d Calculate (approx.) the energy provided by the cell. ...
... cathode half-cell had decreased to .50 M a. Calculate the initial cell potential b. Calculate the cell potential at time, t. c. Calculate the total charge provided by the cell. d Calculate (approx.) the energy provided by the cell. ...
Electrolysis of water
Electrolysis of water is the decomposition of water (H2O) into oxygen (O2) and hydrogen gas (H2) due to an electric current being passed through the water.This technique can be used to make hydrogen fuel (hydrogen gas) and breathable oxygen; though currently most industrial methods make hydrogen fuel from natural gas instead.