Chap 2.1 Notes - Nature of Matter
... 2) Ionic – attractions between oppositely charged ions – involves the gain or lose of electrons. ...
... 2) Ionic – attractions between oppositely charged ions – involves the gain or lose of electrons. ...
Paper
... Steam distillation, using an apparatus like that shown, is a technique used to isolate an organic substance from plant material. The principle of this technique is that the boiling point of a mixture of two immiscible liquids is below the boiling points of both pure liquids. This allows the organic ...
... Steam distillation, using an apparatus like that shown, is a technique used to isolate an organic substance from plant material. The principle of this technique is that the boiling point of a mixture of two immiscible liquids is below the boiling points of both pure liquids. This allows the organic ...
Word and Skeleton Equations
... a) List all the reactants in this reaction. ___________________________________ b) List all the products in this reaction. ___________________________________ c) What is the purpose of the arrow in the word equation? _________________________________________________________________ 2. Write word equ ...
... a) List all the reactants in this reaction. ___________________________________ b) List all the products in this reaction. ___________________________________ c) What is the purpose of the arrow in the word equation? _________________________________________________________________ 2. Write word equ ...
Sample Exam 1
... 1. When methane is burned with oxygen, the products are carbon dioxide and water. If you produce 36 g of water and 44 grams of carbon dioxide from 16 grams of methane, how many grams of oxygen were needed for the reaction? a) 64 g b) 80 g c) 32 g d) 96 g 2. Which of the following is not composed of ...
... 1. When methane is burned with oxygen, the products are carbon dioxide and water. If you produce 36 g of water and 44 grams of carbon dioxide from 16 grams of methane, how many grams of oxygen were needed for the reaction? a) 64 g b) 80 g c) 32 g d) 96 g 2. Which of the following is not composed of ...
CHM 111: General Physical Chemistry 3 Units
... Historical development of the atom: definition of atoms, Daltons atomic theory, relative atomic masses. Fundamental particles of the atom and atomic structure. Modern electronic theory of atoms; electronic configuration of the elements. Periodicity of the elements. Radioactivity: Stoichiometry: mole ...
... Historical development of the atom: definition of atoms, Daltons atomic theory, relative atomic masses. Fundamental particles of the atom and atomic structure. Modern electronic theory of atoms; electronic configuration of the elements. Periodicity of the elements. Radioactivity: Stoichiometry: mole ...
Ch. 2 - Ltcconline.net
... B. Water’s polarity leads to H bonding C. Hydrogen bonds make liquid water cohesive 1. H bonds last for only a few trillionths of a second 2. cohesion and adhesion 3. surface tension D. Hydrogen bonds of water moderate temperature 1. heat 2. temperature E. Ice is less dense than water F. water is a ...
... B. Water’s polarity leads to H bonding C. Hydrogen bonds make liquid water cohesive 1. H bonds last for only a few trillionths of a second 2. cohesion and adhesion 3. surface tension D. Hydrogen bonds of water moderate temperature 1. heat 2. temperature E. Ice is less dense than water F. water is a ...
(activity) of hydrogen ions
... the pH changes by 1 for every power of 10 change in [H+]. – A solution of pH 3 has an H+ concentration 10 times that of a solution of pH 4. The pH scale ranges from 0 to 14. – However, pH values less than 0 and greater than 14 have been observed in very rare concentrated solutions. ...
... the pH changes by 1 for every power of 10 change in [H+]. – A solution of pH 3 has an H+ concentration 10 times that of a solution of pH 4. The pH scale ranges from 0 to 14. – However, pH values less than 0 and greater than 14 have been observed in very rare concentrated solutions. ...
snc 2do unit: chemistry unit test review questions
... 4. Aluminum and oxygen react to form a compound. a) What is the name of the compound formed? b) What is the formula of the compound? c) What type of reaction is this? 5. Identify the type of reaction, and write a balanced chemical equation for: A) zinc + iron (III) nitrate -------> ________ + ______ ...
... 4. Aluminum and oxygen react to form a compound. a) What is the name of the compound formed? b) What is the formula of the compound? c) What type of reaction is this? 5. Identify the type of reaction, and write a balanced chemical equation for: A) zinc + iron (III) nitrate -------> ________ + ______ ...
Determination of electrochemical equivalent of copper and
... Nd – the number of dissociated particles N – overall number of dissolved particles If 0,8 < α < 1 the electrolyte is called strong and it means that almost all dissolved particles were dissociated. For middle electrolytes α = 0,5 and the number of particles dissociated and not dissociated is similar ...
... Nd – the number of dissociated particles N – overall number of dissolved particles If 0,8 < α < 1 the electrolyte is called strong and it means that almost all dissolved particles were dissociated. For middle electrolytes α = 0,5 and the number of particles dissociated and not dissociated is similar ...
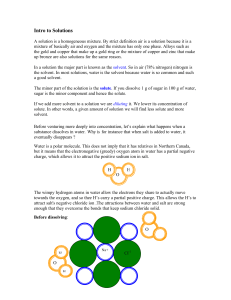
Solutions Intro
... up bronze are also solutions for the same reason. In a solution the major part is known as the solvent. So in air (78% nitrogen) nitrogen is the solvent. In most solutions, water is the solvent because water is so common and such a good solvent. The minor part of the solution is the solute. If you d ...
... up bronze are also solutions for the same reason. In a solution the major part is known as the solvent. So in air (78% nitrogen) nitrogen is the solvent. In most solutions, water is the solvent because water is so common and such a good solvent. The minor part of the solution is the solute. If you d ...
ELECTROLYSIS G10 worksheet for Electrloysis
... ________________________________________________________________________ ________________________________________________________________________ ...
... ________________________________________________________________________ ________________________________________________________________________ ...
biol 1406 chapter 3: water
... Determine if the statement is true. If it is not, rewrite the italicized part to make it true. 1. An element is a substance that can be broken down into simpler substances. ______________________ 2. On Earth, 90 elements occur naturally. ________________________________________ 3. Only four elements ...
... Determine if the statement is true. If it is not, rewrite the italicized part to make it true. 1. An element is a substance that can be broken down into simpler substances. ______________________ 2. On Earth, 90 elements occur naturally. ________________________________________ 3. Only four elements ...
water, h2o
... unique mode of transport in water and, by extension, in other highly connected hydrogen bonding systems. The Grotthuss mechanism involves a simple shift of hydrogen bonds to effectively relocate a net protonic charge from one position to another without significantly moving the mass of the proton. I ...
... unique mode of transport in water and, by extension, in other highly connected hydrogen bonding systems. The Grotthuss mechanism involves a simple shift of hydrogen bonds to effectively relocate a net protonic charge from one position to another without significantly moving the mass of the proton. I ...
AP Biology chap 2 HW - yhs
... different chemical properties, because they have different atomic numbers. b. the same chemical properties, because they have the same number of valence electrons. c. different chemical properties, because they differ in their number of protons and electrons. d. the same chemical properties, because ...
... different chemical properties, because they have different atomic numbers. b. the same chemical properties, because they have the same number of valence electrons. c. different chemical properties, because they differ in their number of protons and electrons. d. the same chemical properties, because ...
17. Electrogravimetric determination of copper in alloys
... electrochemically plated with copper, then – with nickel (to fill the metal pores) and then placed in a silver bath. Afterwards, the statuette is electrochemically coated with 24-karat gold. The quantity of gold can be determined by weighing the statuette before and after the final stage of electro ...
... electrochemically plated with copper, then – with nickel (to fill the metal pores) and then placed in a silver bath. Afterwards, the statuette is electrochemically coated with 24-karat gold. The quantity of gold can be determined by weighing the statuette before and after the final stage of electro ...
Document
... Simple chemical reactions Chemical reactions In a chemical reaction a new substance is always formed. Most chemical changes are not easily reversed; they are irreversible. In a physical change no new substance is formed. Melting and evaporation are examples of physical changes. Physical changes are ...
... Simple chemical reactions Chemical reactions In a chemical reaction a new substance is always formed. Most chemical changes are not easily reversed; they are irreversible. In a physical change no new substance is formed. Melting and evaporation are examples of physical changes. Physical changes are ...
Electrolysis of water
Electrolysis of water is the decomposition of water (H2O) into oxygen (O2) and hydrogen gas (H2) due to an electric current being passed through the water.This technique can be used to make hydrogen fuel (hydrogen gas) and breathable oxygen; though currently most industrial methods make hydrogen fuel from natural gas instead.