* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Lecture 1.1 Some preliminary chemistry knowledge, ppt file
Aromaticity wikipedia , lookup
Homoaromaticity wikipedia , lookup
Metastable inner-shell molecular state wikipedia , lookup
Electrochemistry wikipedia , lookup
Atomic orbital wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Electrolysis of water wikipedia , lookup
Chemical bond wikipedia , lookup
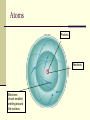
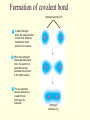
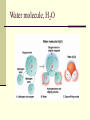
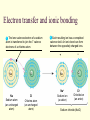
CS882, Fall 2006 Lecture 1.1 Refresh your high school chemistry Atoms and molecules Atoms Protons Neutrons Electrons (much smaller) orbiting around the nucleus Atom models Electron-shell diagrams of the first 18 elements in the periodic table Hydrogen 1H Atomic mass 2 He 4.00 First shell Atomic number Helium 2He Element symbol Electron-shell diagram Lithium 3Li Beryllium 4Be Sodium 11Na Magnesium 12Mg Boron 3B Carbon 6C Nitrogen 7N Silicon 14Si Phosphorus 15P Oxygen 8O Fluorine 9F Neon 10Ne Sulfur 16S Chlorine 17Cl Argon 18Ar Second shell Third shell Aluminum 13Al Formation of covalent bond Hydrogen atoms (2 H) 1 In each hydrogen atom, the single electron is held in its orbital by its attraction to the proton in the nucleus. 2 When two hydrogen atoms approach each other, the electron of each atom is also attracted to the proton in the other nucleus. 3 The two electrons become shared in a covalent bond, forming an H2 molecule. + + + + + + Hydrogen molecule (H2) Water molecule, H2O Electron transfer and ionic bonding 1 The lone valence electron of a sodium 2 Each resulting ion has a completed atom is transferred to join the 7 valence electrons of a chlorine atom. valence shell. An ionic bond can form between the oppositely charged ions. Na Na Sodium atom (an uncharged atom) Cl Cl Chlorine atom (an uncharged atom) + – Na Cl Na+ Sodium ion (a cation) Cl– Chloride ion (an anion) Sodium chloride (NaCl) Sodium chloride crystal Na+ Cl- Hydrogen bond – + H Water (H2O) O H + – Ammonia (NH3) N H H + H + + A hydrogen bond results from the attraction between the partial positive charge on the hydrogen atom of water and the partial negative charge on the nitrogen atom of ammonia. Hydrogen bond Walking on water