C2 Revision Quick Questions FT
... 3. Elements in group 7 form ions with what charge? 4. Elements in group 3 form ions with what charge? 5. Ionic compounds are held together by strong E _ _ _ _ _ _ _ _ _ _ _ C forces in all directions between oppositely charged ions. 6. Under what 2 conditions will ionic compounds conduct electricity ...
... 3. Elements in group 7 form ions with what charge? 4. Elements in group 3 form ions with what charge? 5. Ionic compounds are held together by strong E _ _ _ _ _ _ _ _ _ _ _ C forces in all directions between oppositely charged ions. 6. Under what 2 conditions will ionic compounds conduct electricity ...
C2 revision slides V3 + questions + MS – F
... 3. Elements in group 7 form ions with what charge? 4. Elements in group 3 form ions with what charge? 5. Ionic compounds are held together by strong E _ _ _ _ _ _ _ _ _ _ _ C forces in all directions between oppositely charged ions. 6. Under what 2 conditions will ionic compounds conduct electricity ...
... 3. Elements in group 7 form ions with what charge? 4. Elements in group 3 form ions with what charge? 5. Ionic compounds are held together by strong E _ _ _ _ _ _ _ _ _ _ _ C forces in all directions between oppositely charged ions. 6. Under what 2 conditions will ionic compounds conduct electricity ...
+ CuO Cu + O
... 5- A chemical process in which an atom of the element gains one electron or more. (……………………………………….) 6- The breaking up of bonds between molecules of the reactants and the formation of new bonds between the molecules of the products. ...
... 5- A chemical process in which an atom of the element gains one electron or more. (……………………………………….) 6- The breaking up of bonds between molecules of the reactants and the formation of new bonds between the molecules of the products. ...
Powerpoint File
... hydrophilic (i.e., water-liking), while those that do not dissolve in water are hydrophobic (i.e., water-hating). ...
... hydrophilic (i.e., water-liking), while those that do not dissolve in water are hydrophobic (i.e., water-hating). ...
Final Review
... a. Gases are compressible b. Gases can expand c. Gases can spontaneously diffuse d. Gases are very dense e. Gases have high fluidity 2. When 45.3 grams of C3H8 reacts with oxygen gas, how many liters of CO2 are produced if the reaction is carried out at 56°C and a pressure of 780 mmHg? A. 3.1 B. 81 ...
... a. Gases are compressible b. Gases can expand c. Gases can spontaneously diffuse d. Gases are very dense e. Gases have high fluidity 2. When 45.3 grams of C3H8 reacts with oxygen gas, how many liters of CO2 are produced if the reaction is carried out at 56°C and a pressure of 780 mmHg? A. 3.1 B. 81 ...
science background - CMA
... What does it mean for a solution to be acidic or basic? An acid is a substance that donates hydrogen ions. Because of this, when an acid is dissolved in water, the balance between hydrogen ions and hydroxide ions is shifted. Now there are more hydrogen ions than hydroxide ions in the solution. This ...
... What does it mean for a solution to be acidic or basic? An acid is a substance that donates hydrogen ions. Because of this, when an acid is dissolved in water, the balance between hydrogen ions and hydroxide ions is shifted. Now there are more hydrogen ions than hydroxide ions in the solution. This ...
WS-11-1
... 14. 23.9%, 1.64 m, XCitric acid = 0.0287, 4.11 N 16a. ΔHhyd for CsI = -571 kJ , ΔHhyd for CsOH = -796 kJ 16b. The enthalpy of hydration for CsOH is ore exothermic, indicating a stronger (more stable, lower energy) bond 17. Both are ionic, although the larger charge of the Al3+ ion gives it a greater ...
... 14. 23.9%, 1.64 m, XCitric acid = 0.0287, 4.11 N 16a. ΔHhyd for CsI = -571 kJ , ΔHhyd for CsOH = -796 kJ 16b. The enthalpy of hydration for CsOH is ore exothermic, indicating a stronger (more stable, lower energy) bond 17. Both are ionic, although the larger charge of the Al3+ ion gives it a greater ...
Semester 1 Study Guide – Chemistry
... b. That has the lowest coefficient in the balanced equation c. That has the lowest molar mass d. That is left over after the reaction has gone to completion e. None of the above. ...
... b. That has the lowest coefficient in the balanced equation c. That has the lowest molar mass d. That is left over after the reaction has gone to completion e. None of the above. ...
C2 Additional Chemistry Thursday 14 May
... Can you…? Use the state symbols in equations - (s), (l), (g) and (aq). Describe how soluble salts can be made by reacting acids with metals, insoluble bases and alkalis Describe how salt solutions can be crystallised to produce solid salts. Insoluble salts can be made by mixing certain salts in solu ...
... Can you…? Use the state symbols in equations - (s), (l), (g) and (aq). Describe how soluble salts can be made by reacting acids with metals, insoluble bases and alkalis Describe how salt solutions can be crystallised to produce solid salts. Insoluble salts can be made by mixing certain salts in solu ...
Practice MSL Multiple Choice 1. Compared to the charge and mass
... 116. Salt A and salt B were dissolved separately in 100 mL beakers of water. The water temperatures were measured and recorded as shown in the table below: Salt A: initial water temp. 25.1°C final water temp. 30.2°C Salt B: initial water temp. 25.1°C final water temp. 20.0°C Which statement is a co ...
... 116. Salt A and salt B were dissolved separately in 100 mL beakers of water. The water temperatures were measured and recorded as shown in the table below: Salt A: initial water temp. 25.1°C final water temp. 30.2°C Salt B: initial water temp. 25.1°C final water temp. 20.0°C Which statement is a co ...
Student Notes
... 10e– + 16H+(aq) + 2MnO4– (aq) 2Mn2+(aq) + 8H2O(l) 5C2O42– (aq) 10CO2(g) + 10e– • Now add the reactions and simplify. 16 H+1(aq) + 2 MnO4–1 (aq) + 5 C2O42– (aq) 2 Mn2+(aq) + 8 H2O(l) + 10 CO2(g) • The equation is now balanced! • Note that all of the electrons have cancelled out ! Balancing Equa ...
... 10e– + 16H+(aq) + 2MnO4– (aq) 2Mn2+(aq) + 8H2O(l) 5C2O42– (aq) 10CO2(g) + 10e– • Now add the reactions and simplify. 16 H+1(aq) + 2 MnO4–1 (aq) + 5 C2O42– (aq) 2 Mn2+(aq) + 8 H2O(l) + 10 CO2(g) • The equation is now balanced! • Note that all of the electrons have cancelled out ! Balancing Equa ...
General CHemistry Unit 2 Homework Notes
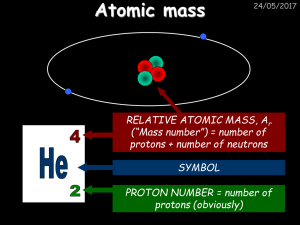
... A neutron has no charge and a relative mass of one. TOPIC TWO: COMPOUNDS & BONDING (PAGE 2) Subscripts in a chemical formula represent the relative number of each type of atom. The subscript always follows the symbol for the element. Example: In a water molecule, H2O, there are 2 hydrogen atoms and ...
... A neutron has no charge and a relative mass of one. TOPIC TWO: COMPOUNDS & BONDING (PAGE 2) Subscripts in a chemical formula represent the relative number of each type of atom. The subscript always follows the symbol for the element. Example: In a water molecule, H2O, there are 2 hydrogen atoms and ...
1.2 Properties and Changes of Matter
... Salt melts (and freezes) at 804°C Oxygen freezes (and melts) at -218°C ...
... Salt melts (and freezes) at 804°C Oxygen freezes (and melts) at -218°C ...
Lab Stuff - WW-P 4
... Calculate the density of an object that has a mass of 38.45 g and occupies 24.7 mL. ...
... Calculate the density of an object that has a mass of 38.45 g and occupies 24.7 mL. ...
NOTES CHEMICAL REACTIONS:
... strong enough to replace the other one. If not, then no reaction will occur ...
... strong enough to replace the other one. If not, then no reaction will occur ...
Document
... Use the equation to explain why the change of iron(III) oxide to iron is a reduction reaction. ...
... Use the equation to explain why the change of iron(III) oxide to iron is a reduction reaction. ...
Name: Date: AP Chemistry/Chemistry 145 Summer Assignment
... What mass of carbon dioxide gas is produced by the reaction of 0.250 moles of calcium carbonate? ...
... What mass of carbon dioxide gas is produced by the reaction of 0.250 moles of calcium carbonate? ...
Units 3 and 4 Revision
... (b) stored under oil. Q5. What happens to the melting point of the elements in group 7 (the halogens) as you go the group? Answers:- Q3. Lithium. Q4. (a) The elements in group 1 react with water to form an ...
... (b) stored under oil. Q5. What happens to the melting point of the elements in group 7 (the halogens) as you go the group? Answers:- Q3. Lithium. Q4. (a) The elements in group 1 react with water to form an ...
Electrolysis of water
Electrolysis of water is the decomposition of water (H2O) into oxygen (O2) and hydrogen gas (H2) due to an electric current being passed through the water.This technique can be used to make hydrogen fuel (hydrogen gas) and breathable oxygen; though currently most industrial methods make hydrogen fuel from natural gas instead.