* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Bio 102 Lecture - chapter 2 The Chemical Basis of Life
Metastable inner-shell molecular state wikipedia , lookup
Electrochemistry wikipedia , lookup
History of chemistry wikipedia , lookup
Biochemistry wikipedia , lookup
Properties of water wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Water pollution wikipedia , lookup
Freshwater environmental quality parameters wikipedia , lookup
Electronegativity wikipedia , lookup
Atomic orbital wikipedia , lookup
Extended periodic table wikipedia , lookup
Atomic nucleus wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Metalloprotein wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
Resonance (chemistry) wikipedia , lookup
Hydrogen atom wikipedia , lookup
Artificial photosynthesis wikipedia , lookup
Molecular orbital diagram wikipedia , lookup
Hydrogen bond wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Metallic bonding wikipedia , lookup
Water splitting wikipedia , lookup
Electrolysis of water wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Electron configuration wikipedia , lookup
History of molecular theory wikipedia , lookup
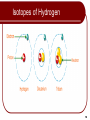
Chapter 2 The Chemical Basis of Life 1 Outline Chemical Elements Atoms Periodic Table Isotopes Electrons and Energy Molecules and Compounds Chemical Bonding Atomic Mass and Atomic Number Ionic and Covalent Hydrogen Properties of Water Acids and Bases 2 Chemical Elements Matter: Matter is defined as anything that has mass and occupies space Matter exists in three states: solid, liquid, and gas All matter (both living and non-living) is composed of 92 naturally-occurring elements 98% of body weight of organisms are primarily composed of six elements (carbon, hydrogen, nitrogen, oxygen, phosphorus, and sulfur—acronym CHNOPS) make up 98% of the body weight of organisms. 3 Atomic Structure Atom is the smallest unit of an element Atoms composed of subatomic particles: Protons - positive charge; found in the nucleus Neutrons - no charge; found in the nucleus Electrons - negative charge; found in electron shell Atoms contain specific numbers of protons, neutrons, and electrons. 4 Subatomic Particles Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. = proton = neutron = electron a. b. Subatomic Particles Particle Electric Charge Atomic Mass Unit (AMU) Location Proton +1 1 Nucleus Neutron 0 1 Nucleus Electron –1 0 Electron shell c. 5 Atomic Symbols Each element is represented by one or two letters to give them a unique atomic symbol e.g. H = hydrogen, Na = Sodium, C = Carbon Atomic number = proton number = Electron number Atomic mass or mass number = protons and neutrons The atomic number is above the atomic symbol and the atomic mass is below the atomic symbol Atomic Number Mass Number 6 12 6 Carbon C Atomic Symbo l 6 Periodic Table Elements grouped in periodic table based on characteristics Vertical columns = groups; chemically similar Horizontal rows = periods; Atomic mass increases as you move down a group or across a period. 7 Periodic Table Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. VIII I 1 atomic number H atomic symbol He atomic mass II III IV V VI VII 4.003 3 4 5 6 7 8 9 10 Li Be B C N O F Ne 6.941 9.012 10.81 12.01 14.01 16.00 19.00 20.18 1 12 13 14 15 16 17 18 Na Mg Al Si P S Cl Ar 22.99 24.31 26.98 28.09 30.97 32.07 35.45 39.95 19 20 31 32 33 34 35 36 K Ca Ga Ge As Se Br Kr 39.10 40.08 69.72 72.59 74.92 78.96 79.90 83.60 1.008 Periods 2 Groups 8 Isotopes Isotopes: Atoms of the same element with a differing numbers of neutrons (and therefore have different atomic masses). e.g. see carbon below Some isotopes spontaneously decay Radioactive Give Can off energy in the form of rays. be used as tracers Mutagenic 12 6 C Carbon 12 – Can cause cancer 13 6 C Carbon 13 14 6 C Carbon 14 9 Isotopes of Hydrogen 10 Electrons and Energy Atoms normally have as many electrons as protons. Opposite charges balance leaving atom neutral Electrons revolve around nucleus in different shells, labeled from the innermost shell as K, L, M, N, etc. 11 12 Each shell can have a certain number of electrons. The K-shell can have 2 Electrons, the L-shell, 8, the M-shell 18, Nshell 32. This is calculated by using the formula 2N², where N=1 for the K shell, N=2 for the L shell, N=3 for the M shell, etc. 13 Different shells in the atom 14 Animation Please note that due to differing operating systems, some animations will not appear until the presentation is viewed in Presentation Mode (Slide Show view). You may see blank slides in the “Normal” or “Slide Sorter” views. All animations will appear after viewing in Presentation Mode and playing each animation. Most animations will require the latest version of the Flash Player, which is available at http://get.adobe.com/flashplayer. 15 Arrangements of Electrons in an Atom 16 The outermost electron shell determines the reactivity of the element 17 The Octet Rule for Distribution of Electrons 8-electron configuration is stable because this atom is having 8 valence electrons. 18 Chemical Bonding If 3 or less electrons in the outer most shell – Tendency to donate electrons. If 5 or more electrons in the outer most shell – Tendency to receive electrons. A ‘chemical bond’ the force of attraction between atoms to attain stability. Bonds between atoms are caused by electrons in outermost shells The process of bond formation is called a reaction. 19 Compounds and Molecules Compound - when atoms of two or more different elements bond together CO2, H2O, C6H12O6, etc. Characteristics dramatically different from constituent elements Molecule and compound is used interchangeably In Biology molecule is used e.g. molecule of water (H2O) molecule of glucose (C6H12O6 ) 20 Compounds and Molecules Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. one molecule indicates 6 atoms of carbon indicates 12 atoms of hydrogen indicates 6 atoms of oxygen 21 Types of Bonds: Ionic Bonding Ionic bond - forms when electrons are transferred from one atom to another atom. Octet rule – atoms lose or gain electrons to fill their outer shells and become more stable Atoms “want” 8 electrons in outer shell If have < 4, desire to donate electrons If have > 4, desire to receive electrons Consider two elements from opposite ends of periodic table Element from right side: Has 7 electrons in outer shell “Desperately wants” one more (7+1=8) Element from left side: Has only 1 electron in outer shell “Desperately wants” to donate it (1-1=0=8) 22 Types of Bonds: Ionic Bond Example Sodium (Na): Has only 1 electron in its outermost shell Chlorine (Cl): Has 7 electrons in its outermost shell In a reaction between Na and Cl Na loses an electron and becomes a positive ion (Na+) - CATION Cl gains an electron and becomes a negative ion (Cl-) - ANION Attraction of oppositely charged ions holds the two atoms together in an ionic bond. 23 Formation of Sodium Chloride Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Na Cl sodium atom (Na) chlorine atom (Cl) – + Na sodium ion (Na+) − Na+ Cl Cl chloride ion (Cl−) sodium chloride (NaCl) a. b. (Crystals): © Charles M. Falco/Photo Researchers, Inc.; (Salt shaker): © Erica S. Leeds 24 25 Types of Bonds: Covalent Bonds Covalent bonds result when two atoms share electrons so each atom has an octet of electrons in the outer shell (in the case of hydrogen, 2 electrons). When atoms are horizontally closer together in the periodic table The electrons are not permanently transferred from one atom to the other like in NaCl A pair of electrons from the outer shell will “time share” with one atom and then the other This also causes the atoms to remain together Known as covalent bonding. 26 Covalently Bonded Molecules Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. The structural formula of a molecule indicates a shared pair of electrons by a line between the two atoms e.g. single covalent bond (H–H), double covalent bond (O=O), and triple covalent bond (N = N). Structural Formula Electron Model H H H Molecular Formula H2 H a. Hydrogen gas O O O O O2 b. Oxygen gas H H H C H H C H CH4 H H c. Methane 27 Nonpolar Covalent Bonds In non-polar covalent bonds, sharing of electrons is equal. 28 Polar Covalent Bonds With polar covalent bonds, the sharing of electrons is unequal. In H2O - sharing of electrons by oxygen and hydrogen is not equal; the oxygen atom therefore assumes a partial negative charge. 29 Animation Please note that due to differing operating systems, some animations will not appear until the presentation is viewed in Presentation Mode (Slide Show view). You may see blank slides in the “Normal” or “Slide Sorter” views. All animations will appear after viewing in Presentation Mode and playing each animation. Most animations will require the latest version of the Flash Player, which is available at http://get.adobe.com/flashplayer. 30 Types of Bonds: Hydrogen Bonds Water (H2O or H–O–H) is a polar molecule H’s become slightly +, O slightly – The H’s of water molecules are attracted to the negative parts of the oxygen molecules and form hydrogen bond. This bond is a weak bond because of weak attractive force between the slightly positive charge of the hydrogen atom of one molecule and slightly negative charge of another atom Easily broken. Found in water, proteins and nucleic acids 31 Hydrogen Bonds 32 Water Molecule Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Electron Model Ball-and-stick Model Space-filling Model O Oxygen attracts the shared electrons and is partially negative. – O H O H H 104.5° H H + H + Hydrogens are partially positive. a. Water (H2O) + H – O H + hydrogen bond b. Hydrogen bonding between water molecules 33 The Chemistry of Water: Heat Capacity The specific heat is the amount of heat (energy) needed to raise a one gram of a substance by one degree Celsius. Water has a high heat capacity (high specific heat) due to the hydrogen bonding. More heat is required to raise water’s temperature than most other liquids. 34 Properties of Water: High Heat of Vaporization Heat of Vaporization is the quantity of heat a liquid must absorb to be converted from the liquid to the gaseous state. A substance which has a high heat of vaporization requires more heat to turn from liquid to vapor. Large numbers of hydrogen bonds must be broken to evaporate water. This is why sweating cools. 35 Evaporative Cooling of Animals Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Calories of Heat Energy / g 800 Gas 600 540 calories 400 200 Liquid 80 calories Solid 0 freezing occurs 0 evaporation occurs 20 40 60 80 100 120 Temperature (°C) a. Calories lost when 1 g of liquid water freezes and calories required when 1 g of liquid water evaporates. b. Bodies of organisms cool when their heat is used to evaporate water. © Grant Taylor/Getty Images 36 Heat Content of Water at Various Temperatures Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Calories of Heat Energy / g 800 Gas 600 540 calories 400 200 0 Liquid 80 calories Solid freezing occurs 0 evaporation occurs 20 40 60 80 100 120 Temperature (°C) a. Calories lost when 1 g of liquid water freezes and calories required when 1 g of liquid water evaporates. 37 Properties of Water: Water as a Solvent Solutions consist of: A solvent (the most abundant part) and A solute (less abundant part) that is dissolved in the solvent Hydrophilic molecules are soluble in/ interact with/ dissolve in water. Polar compounds and Ionic readily dissolve in water by forming hydrogen bonds. Example: glucose, salt A hydrophobic molecules are insoluble (do not dissolve) in water. Example: oils or fats 38 Properties of Water: Water as a Solvent Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. H O O H - An ionic salt dissolves in water. Na+ H O H - - O H H + + H O H H H + Cl– + H H O 39 Properties of Water: Water as a Solvent Ammonia (NH3) H O H + A polar molecule dissolves in water. - + N + H H H H O H + - - - O H H O H H 40 Properties of Water: Uniqueness of Ice Frozen water (ice) is less dense than liquid water so ice floats in water. Otherwise, oceans and deep lakes would fill with ice from the bottom up Ice acts as an insulator on top of a frozen body of water Melting ice draws heat from the environment 41 Water as a Transport Medium Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Water evaporates, pulling the water column from the roots to the leaves. H2O Water molecules cling together and adhere to sides of vessels in stems. Water enters a plant at root cells. H2O 42 A Pond in Winter Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. ice layer Protists provide food for fish. River otters visit ice-covered ponds. Aquatic insects survive in air pockets. Freshwater fish take oxygen from water. Common frogs and pond turtles hibernate. 43 Properties of Water: Cohesion & Adhesion Cohesion – When hydrogen bonds hold water molecules tightly together, it is called cohesion. Adhesion – When water molecules adhere to polar surfaces (due to hydrogen bonds), it is called adhesion. Cohesion and adhesion allow water be drawn many meters up a tree in a tubular vessel 44 Properties of Water: Surface Tension High Surface Tension Water molecules at surface hold more tightly than below surface Allows small non-polar objects (like water strider) to sit on top of water 45 When water ionizes or dissociates, it releases a small but equal number of hydrogen (H+) ions and hydroxide (OH-) ions H–O–H H+ + OH- 46 Acids and Bases Acids donate hydrogen ions Dissociate in water and release hydrogen ions (H+) e.g. Hydrochloric acid (HCl) HCl → H+ + Cl Bases accepts hydrogen ions (H+) or release hydroxide ions (OH-) e.g. Sodium hydroxide (NaOH) In water, it dissociates into Na+ and OH47 Acid and Base 48 pH Scale The pH is a mathematical way of indicating the number of H+ ions in a solution. pH scale used to indicate acidity and alkalinity of a solution. Values range from 0 -14; 0 to 6 = Acidic; 7 = Neutral; 8 to 14 = Basic (or alkaline) Each unit in pH represents a change of 10X pH of 4 is 10X as acidic as pH of 5 pH of 10 is 100X more basic than pH of 8 49 The pH Scale Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. basic acidic H+ Ion Concentration 10 0 10–1 10–2 10–3 10–4 10–5 10–6 10–7 10–8 10–9 10–10 10–11 10–12 10–13 10–14 pH value 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Examples hydrochloric acid stomach acid, lemon juice vinegar, cola, beer tomatoes black coffee urine pure water seawater baking soda Great Salt Lake household ammonia household bleach sodium hydroxide 50 Buffers and pH When H+ is added to pure water at pH 7, pH goes down and water becomes acidic When OH- is added to pure water at pH 7, pH goes up and water becomes alkaline Buffers are solutes in water that resist change in pH When H+ is added, buffer may absorb, or counter by adding OH- When OH- is added, buffer may absorb, or counter by adding H+ 51 Buffers in Biology Health of organisms requires maintaining pH of body fluids within narrow limits Human blood normally 7.4 (slightly alkaline) Many foods and metabolic processes add or subtract H+ or OH- ions Reducing blood pH to 7.0 results in acidosis Increasing blood pH to 7.8 results in alkalosis Both life threatening situations Bicarbonate ion (-HCO3) in blood buffers pH to 7.4 52 Review Chemical Elements Atoms Isotopes Molecules and Compounds Chemical Bonding Ionic and Covalent Hydrogen Properties of Water Acids and Bases 53 Chapter 2: pp. 21-35 Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Na Cl sodium atom (Na) chlorine atom (Cl) – + Na sodium ion (Na+) Na+ Cl– 10th Edition Sylvia S. Mader Basic Chemistry BIOLOGY Cl chloride ion (Cl–) sodium chloride (NaCl) a. b. (Crystals): © Charles M. Falco/Photo Researchers, Inc.; (Salt shaker): © Erica S. Leeds PowerPoint® Lecture Slides are prepared by Dr. Isaac Barjis, Biology Instructor Copyright © The McGraw Hill Companies Inc. Permission required for reproduction or display 54