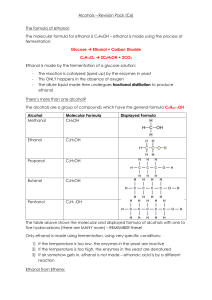
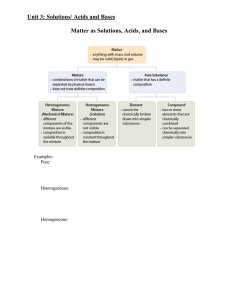
Document
... The Earth is the only water rich planet in the solar system. Its appearance from space is dominated by the blue colour of thick layers of water, the white reflection of sunlight from ice crystals and water droplets in clouds, the brown landscape shaped by water and the green of water rich plants. Wa ...
... The Earth is the only water rich planet in the solar system. Its appearance from space is dominated by the blue colour of thick layers of water, the white reflection of sunlight from ice crystals and water droplets in clouds, the brown landscape shaped by water and the green of water rich plants. Wa ...
Solutions
... One of the main difficulties in the study of solutions is the wide variety of variables and units used to specify concentration (in the case of ideal mixtures, only molar fractions, and sometimes mass fractions, were common). Generically speaking, concentrations in a mixture express the quantitative ...
... One of the main difficulties in the study of solutions is the wide variety of variables and units used to specify concentration (in the case of ideal mixtures, only molar fractions, and sometimes mass fractions, were common). Generically speaking, concentrations in a mixture express the quantitative ...
Name: Period:______ Let`s make some sandwiches! Introduction: If
... Name:______________________ Period:_________ Let’s make some sandwiches! Introduction: If a sandwich shop runs out of bread, the shop closes down. No more sandwiches can be fully made without ordering more bread from a bakery. A similar thing happens in a chemical reaction. If there are fixed amount ...
... Name:______________________ Period:_________ Let’s make some sandwiches! Introduction: If a sandwich shop runs out of bread, the shop closes down. No more sandwiches can be fully made without ordering more bread from a bakery. A similar thing happens in a chemical reaction. If there are fixed amount ...
Mechanisms and energetics of surface reactions at the copper
... In order to make a critical analysis of the discussion of corrosion of copper in pure anoxic water it is necessary to understand the chemical reactivity at the copper-water interface. Even though the most fundamental issue, i.e. the nature and existence of a hypothetical product that is thermodynami ...
... In order to make a critical analysis of the discussion of corrosion of copper in pure anoxic water it is necessary to understand the chemical reactivity at the copper-water interface. Even though the most fundamental issue, i.e. the nature and existence of a hypothetical product that is thermodynami ...
الشريحة 1
... molecules are strong enough to pull the ions from their positions in the crystal. Once separated from the crystal, the Na+ and Cl- ion are surrounded by H2O molecules. This type of interaction between solute and solvent molecules are known as solvation. When the solvent is water, the interaction are ...
... molecules are strong enough to pull the ions from their positions in the crystal. Once separated from the crystal, the Na+ and Cl- ion are surrounded by H2O molecules. This type of interaction between solute and solvent molecules are known as solvation. When the solvent is water, the interaction are ...
Ch 12 Solutions
... - Substances will mix and become disordered if no forces prevent them from doing so. - If only London forces are present, then there are no large differences in attractions. The molecules will move freely together as long as T is much less than their boiling points. - The same is true of polar molec ...
... - Substances will mix and become disordered if no forces prevent them from doing so. - If only London forces are present, then there are no large differences in attractions. The molecules will move freely together as long as T is much less than their boiling points. - The same is true of polar molec ...
The Chemistry of Aqueous Systems
... convert the 65 psi into atm (4.42 atm). Rearrange the equation and solve for "X": Boyle's Law is applicable to breathing. Note that just before inspiration that the atmospheric, intrapleural (in between the two membranes around the lungs) and intrapulmonic (in the lungs) pressures are all equal: 760 ...
... convert the 65 psi into atm (4.42 atm). Rearrange the equation and solve for "X": Boyle's Law is applicable to breathing. Note that just before inspiration that the atmospheric, intrapleural (in between the two membranes around the lungs) and intrapulmonic (in the lungs) pressures are all equal: 760 ...
chemistry -- questions -
... __ 23. An atom's atomic number is best described as the number of a) protons it contains. b) neutrons it contains. c) electrons in the outermost shell. d) protons and neutrons it contains. e) protons and electrons it contains. __ 24. An atom's atomic mass is best described as the mass of a) the pro ...
... __ 23. An atom's atomic number is best described as the number of a) protons it contains. b) neutrons it contains. c) electrons in the outermost shell. d) protons and neutrons it contains. e) protons and electrons it contains. __ 24. An atom's atomic mass is best described as the mass of a) the pro ...
الشريحة 1
... molecules are symmetrical enough to cancel much of the weak C-H bond dipoles. The attraction between the polar water molecules and the nonpolar hydrocarbon molecules is not sufficiently strong to allow the formation of a solution. ...
... molecules are symmetrical enough to cancel much of the weak C-H bond dipoles. The attraction between the polar water molecules and the nonpolar hydrocarbon molecules is not sufficiently strong to allow the formation of a solution. ...
C6 Revision Guide - West Derby School
... these substances were inert (unreactive). However, in the 1970s, scientists began to link the ozone depletion with CFCs. CFCs, Ozone and Radicals: In the stratosphere, the UV radiation from the sun breaks down the CFC molecules. This makes highly reactive chlorine atoms. One of these reactive chlori ...
... these substances were inert (unreactive). However, in the 1970s, scientists began to link the ozone depletion with CFCs. CFCs, Ozone and Radicals: In the stratosphere, the UV radiation from the sun breaks down the CFC molecules. This makes highly reactive chlorine atoms. One of these reactive chlori ...
getting started 3.1 hydrocarbons
... 1. A functional group is a structural arrangement of atoms that, because of their electronegativity and bonding type, imparts particular characteristics to the molecule. 2. C=C and C)C bonds are more reactive than C–C bonds because the second and third bonds formed are weaker than the single bonds f ...
... 1. A functional group is a structural arrangement of atoms that, because of their electronegativity and bonding type, imparts particular characteristics to the molecule. 2. C=C and C)C bonds are more reactive than C–C bonds because the second and third bonds formed are weaker than the single bonds f ...
WA AP Chem gas law IMF MC Set C
... B. The intermolecular forces, such as hydrogen bonds, between the H2O (l) molecules require energy to be overcome, and the molecules can only separate to become a gas once these intermolecular forces are overcome. C. Water vapor is hotter than or equal in temperature to water at constant pressure, a ...
... B. The intermolecular forces, such as hydrogen bonds, between the H2O (l) molecules require energy to be overcome, and the molecules can only separate to become a gas once these intermolecular forces are overcome. C. Water vapor is hotter than or equal in temperature to water at constant pressure, a ...
Hydrogen bond dynamics of superheated water and methanol by
... half the average chain-length compared with that of liquid methanol at ambient temperature. Density fluctuations of SCM have also been reported by measurements of small-angle neutron scattering.44 Since most of the results on supercritical fluids are, however, obtained from static non time-resolved ...
... half the average chain-length compared with that of liquid methanol at ambient temperature. Density fluctuations of SCM have also been reported by measurements of small-angle neutron scattering.44 Since most of the results on supercritical fluids are, however, obtained from static non time-resolved ...
Chapter - Archie Main Page
... that was used to heat a volume of water must be removed for it to cool. If a large amount of energy is needed to heat water, the same amount will be have to be taken away to cool it back to the starting temperature. This also explains why water cools more slowly than other substances. Water has a hi ...
... that was used to heat a volume of water must be removed for it to cool. If a large amount of energy is needed to heat water, the same amount will be have to be taken away to cool it back to the starting temperature. This also explains why water cools more slowly than other substances. Water has a hi ...
Preview Sample 2
... You also notice that the electrons in H2 are evenly distributed among the two atoms. Which two types of bonds are represented in these molecules? A. Covalent bonds in NaCl; ionic bonds in H2. B. Covalent bonds in NaCl; covalent bonds in H2. C. Ionic bonds in NaCl; ionic bonds in H2. D. Ionic bonds i ...
... You also notice that the electrons in H2 are evenly distributed among the two atoms. Which two types of bonds are represented in these molecules? A. Covalent bonds in NaCl; ionic bonds in H2. B. Covalent bonds in NaCl; covalent bonds in H2. C. Ionic bonds in NaCl; ionic bonds in H2. D. Ionic bonds i ...
Outline for Unit 1 Solutions, Acid/Base, and Gases
... Molarity is the most common method used for expressing concentration of aqueous solutions. We will use this method for the rest of the unit. If asked for ion molarity, watch your ratio of compound/ion. Concentration of solution vs. Concentration of ions (dissociation/ionization) With strong electro ...
... Molarity is the most common method used for expressing concentration of aqueous solutions. We will use this method for the rest of the unit. If asked for ion molarity, watch your ratio of compound/ion. Concentration of solution vs. Concentration of ions (dissociation/ionization) With strong electro ...
1 Assignment 5 Hydrogen – The Unique Element
... different to those formed with elements further down the periodic table. For example, the halogens form acidic hydrides e.g. HCl, but it should be noted that HF is anomalous in that it is a weak acid. Hydrides of the pnictogens are all Lewis bases and useful ligands in coordination chemistry. Howeve ...
... different to those formed with elements further down the periodic table. For example, the halogens form acidic hydrides e.g. HCl, but it should be noted that HF is anomalous in that it is a weak acid. Hydrides of the pnictogens are all Lewis bases and useful ligands in coordination chemistry. Howeve ...
1 Assignment 4 Hydrogen – The Unique Element
... different to those formed with elements further down the periodic table. For example, the halogens form acidic hydrides e.g. HCl, but it should be noted that HF is anomalous in that it is a weak acid. Hydrides of the pnictogens are all Lewis bases and useful ligands in coordination chemistry. Howeve ...
... different to those formed with elements further down the periodic table. For example, the halogens form acidic hydrides e.g. HCl, but it should be noted that HF is anomalous in that it is a weak acid. Hydrides of the pnictogens are all Lewis bases and useful ligands in coordination chemistry. Howeve ...
Chapter 17 Green chemistry
... A 2NH3(aq) + H2SO4(aq) (NH4)2SO4 (aq) B O2–(aq) + H2O(l) 2OH–(aq) C HSO4–(aq) + H2O(l) H3O+(aq) + SO42–(aq) D Zn(s) + H2SO4(aq) ZnSO4(aq) + H2(g) A10. D. A Brønsted–Lowry reaction requires a proton transfer to occur. Q11. Which of the four equations represents a reaction in which the Fe2+ io ...
... A 2NH3(aq) + H2SO4(aq) (NH4)2SO4 (aq) B O2–(aq) + H2O(l) 2OH–(aq) C HSO4–(aq) + H2O(l) H3O+(aq) + SO42–(aq) D Zn(s) + H2SO4(aq) ZnSO4(aq) + H2(g) A10. D. A Brønsted–Lowry reaction requires a proton transfer to occur. Q11. Which of the four equations represents a reaction in which the Fe2+ io ...
Water - UFMG
... in liquid water have a bond dissociation energy (the energy required to break a bond) of about 20 kJ/mol, compared with 348 kJ/mol for the covalent CXC bond. At room temperature, the thermal energy of an aqueous solution (the kinetic energy of motion of the individual atoms and molecules) is of the ...
... in liquid water have a bond dissociation energy (the energy required to break a bond) of about 20 kJ/mol, compared with 348 kJ/mol for the covalent CXC bond. At room temperature, the thermal energy of an aqueous solution (the kinetic energy of motion of the individual atoms and molecules) is of the ...
Unit C3 - Chemistry In Action
... fat content) 2) Dinosaurs would have drunk the same water you do 3) Water dissolves more substances than any other liquid – most ionic substances are soluble and most covalent substances are insoluble 4) Around 75% of the world’s surface is made of water ...
... fat content) 2) Dinosaurs would have drunk the same water you do 3) Water dissolves more substances than any other liquid – most ionic substances are soluble and most covalent substances are insoluble 4) Around 75% of the world’s surface is made of water ...
Unit C3 - Chemistry in Action
... fat content) 2) Dinosaurs would have drunk the same water you do 3) Water dissolves more substances than any other liquid – most ionic substances are soluble and most covalent substances are insoluble 4) Around 75% of the world’s surface is made of water ...
... fat content) 2) Dinosaurs would have drunk the same water you do 3) Water dissolves more substances than any other liquid – most ionic substances are soluble and most covalent substances are insoluble 4) Around 75% of the world’s surface is made of water ...
Campbell Biology in Focus (Urry) Chapter 2 The Chemical Context
... 57) Which type of bond must be broken for water to vaporize? A) ionic bonds B) both hydrogen bonds and ionic bonds C) polar covalent bonds D) hydrogen bonds E) both polar covalent bonds and hydrogen bonds 58) Temperature usually increases when water condenses. Which behavior of water is most directl ...
... 57) Which type of bond must be broken for water to vaporize? A) ionic bonds B) both hydrogen bonds and ionic bonds C) polar covalent bonds D) hydrogen bonds E) both polar covalent bonds and hydrogen bonds 58) Temperature usually increases when water condenses. Which behavior of water is most directl ...
WEEK 6
... We earlier defined salts as compounds formed between a positive ion other than H+ and a negative ion other than OH-. In pure form salts usually exist as crystalline solids at room temperature. Salts are ionic substance. Those that dissolve in water dissociate into positive and negative ions. Classif ...
... We earlier defined salts as compounds formed between a positive ion other than H+ and a negative ion other than OH-. In pure form salts usually exist as crystalline solids at room temperature. Salts are ionic substance. Those that dissolve in water dissociate into positive and negative ions. Classif ...
Chemistry Fall Final Study Guide Concepts
... 4. What would you observe for H2O(s), H2O(l), H2O(g), and NaCl (aq)? I would observe ice, water, steam (water vapor), and salt water (salt dissolved in water). 5. Using the periodic table, where are the metals and nonmetals? What is hydrogen? Metals are on the left side and the nonmetals are on the ...
... 4. What would you observe for H2O(s), H2O(l), H2O(g), and NaCl (aq)? I would observe ice, water, steam (water vapor), and salt water (salt dissolved in water). 5. Using the periodic table, where are the metals and nonmetals? What is hydrogen? Metals are on the left side and the nonmetals are on the ...
Properties of water
Water (H2O) is the most abundant compound on Earth's surface, covering 70 percent of the planet. In nature, water exists in liquid, solid, and gaseous states. It is in dynamic equilibrium between the liquid and gas states at standard temperature and pressure. At room temperature, it is a tasteless and odorless liquid, nearly colorless with a hint of blue. Many substances dissolve in water and it is commonly referred to as the universal solvent. Because of this, water in nature and in use is rarely pure and some properties may vary from those of the pure substance. However, there are also many compounds that are essentially, if not completely, insoluble in water. Water is the only common substance found naturally in all three common states of matter and it is essential for all life on Earth. Water makes up 55% to 78% of the human body.