Chapter 2_Application Problems
... amu and an abundance of 98.89% while carbon 13 has an isotopic mass of 13.0034 amu and an abundance of 1.11%. Calculate the atomic mass of carbon. ...
... amu and an abundance of 98.89% while carbon 13 has an isotopic mass of 13.0034 amu and an abundance of 1.11%. Calculate the atomic mass of carbon. ...
ATOMS
... • The ATOMIC NUMBER is the number of protons in the nucleus of an atom. For example: Carbon’s atomic number is 6. So, there are 6 protons in the nucleus. Oxygen has an atomic number of 8. • How many protons are there? ...
... • The ATOMIC NUMBER is the number of protons in the nucleus of an atom. For example: Carbon’s atomic number is 6. So, there are 6 protons in the nucleus. Oxygen has an atomic number of 8. • How many protons are there? ...
THE ATOM
... • Isotopes – different atoms of the same element that have the same number of protons but different numbers of neutrons • some isotopes are radioactive – they emit energy when the nucleus of the atom breaks down spontaneously • most radioactive isotopes are not dangerous • to determine if an isotope ...
... • Isotopes – different atoms of the same element that have the same number of protons but different numbers of neutrons • some isotopes are radioactive – they emit energy when the nucleus of the atom breaks down spontaneously • most radioactive isotopes are not dangerous • to determine if an isotope ...
Chapter 3: Mass Relations:
... the mass of a carbon – 12 atom. • The weighted average of the masses of the isotopes of an element create the elements atomic mass. – Remember completing weighted avg??? ...
... the mass of a carbon – 12 atom. • The weighted average of the masses of the isotopes of an element create the elements atomic mass. – Remember completing weighted avg??? ...
Chemistry Calendar Omega 10 10/24 – 10/28 Monday Oct 24
... interpret the symbol for a specific isotope Predict the number of protons, neutrons, and electrons in the most abundant isotope of an atom ...
... interpret the symbol for a specific isotope Predict the number of protons, neutrons, and electrons in the most abundant isotope of an atom ...
AP Exam One Retake Qualifying Assignment
... a mobile phase carries the sample to be separated can be used to separate alcohol from water separates large substances from smaller substances the oil in a water and oil mixture Mercury Glucose C6H12O6 Gatorade cannot be broken down any further by chemical means the atmosphere Use only the best ter ...
... a mobile phase carries the sample to be separated can be used to separate alcohol from water separates large substances from smaller substances the oil in a water and oil mixture Mercury Glucose C6H12O6 Gatorade cannot be broken down any further by chemical means the atmosphere Use only the best ter ...
Chemistry Atomic structure Chapter 4, and Chapter 5, p. 146-148
... When atoms acquire energy their electrons become exited and orbit at a further distance from the nucleus. Bohr assigned each orbit a different value called a quantum number. When the atom returns to its ground state the electrons revert to their closer orbits which release light energy that can be o ...
... When atoms acquire energy their electrons become exited and orbit at a further distance from the nucleus. Bohr assigned each orbit a different value called a quantum number. When the atom returns to its ground state the electrons revert to their closer orbits which release light energy that can be o ...
File
... C. Purpose: provide support & speed reactions D. Enzymes are specialized proteins that function as catalysts for chemical reactions. E. Examples of those important to humans: 1. Digestive enzymes, collagen, etc. Too many to list them all – they make up 15% of your total body mass! ...
... C. Purpose: provide support & speed reactions D. Enzymes are specialized proteins that function as catalysts for chemical reactions. E. Examples of those important to humans: 1. Digestive enzymes, collagen, etc. Too many to list them all – they make up 15% of your total body mass! ...
Chemistry PowerPoint
... a. The total mass of the reactants is greater than the total mass of the products b. The total mass of the reactants is less than the total mass of the products c. The total mass of the reactants equals the total mass of the products d. Mass can be created and destroyed ...
... a. The total mass of the reactants is greater than the total mass of the products b. The total mass of the reactants is less than the total mass of the products c. The total mass of the reactants equals the total mass of the products d. Mass can be created and destroyed ...
Fundamentals of Chemistry
... More on the Atom • An atom is uniquely defined by #p+ = Z = atomic number (see Periodic Table) • In a neutral atom, #p+ = #e-; note that #n is not equal to #p+ nor #e-. • In an atomic ion, #p+ ≠ #e- resulting in a net nonzero charge on the species – Neutral atoms can lose electrons producing a posi ...
... More on the Atom • An atom is uniquely defined by #p+ = Z = atomic number (see Periodic Table) • In a neutral atom, #p+ = #e-; note that #n is not equal to #p+ nor #e-. • In an atomic ion, #p+ ≠ #e- resulting in a net nonzero charge on the species – Neutral atoms can lose electrons producing a posi ...
Chapter 2
... • Dalton’s atomic theory – All matter composed of indivisible atoms • Atom: small particle, retains identity in reactions – Element: type of matter composed of only one kind of atom – Compound: type of matter composed of fixed proportion of 2 or more elements – Chemical reaction: rearrangement of at ...
... • Dalton’s atomic theory – All matter composed of indivisible atoms • Atom: small particle, retains identity in reactions – Element: type of matter composed of only one kind of atom – Compound: type of matter composed of fixed proportion of 2 or more elements – Chemical reaction: rearrangement of at ...
atom
... • Atoms of different elements combine in simple wholenumber ratios to form chemical compounds. ...
... • Atoms of different elements combine in simple wholenumber ratios to form chemical compounds. ...
Multiple Choice Questions
... 17. The amount of energy released from a nuclear reaction is much greater than a chemical reaction because (1) mass is converted into energy (2) energy is converted into mass (3) ionic bonds are broken (4) covalent bonds are broken 18. Which reaction releases the greatest amount of energy per mole o ...
... 17. The amount of energy released from a nuclear reaction is much greater than a chemical reaction because (1) mass is converted into energy (2) energy is converted into mass (3) ionic bonds are broken (4) covalent bonds are broken 18. Which reaction releases the greatest amount of energy per mole o ...
Name Date Period DEFINING THE ATOM Section Review
... Part B True-False Classify each of these statements as always true, AT; sometimes true, ST; or never true, NT. 5. Atoms of one element change into atoms of another element during chemical reactions. 6. Atoms combine in one-to-one ratios to form compounds. 7. Atoms of one element are different from a ...
... Part B True-False Classify each of these statements as always true, AT; sometimes true, ST; or never true, NT. 5. Atoms of one element change into atoms of another element during chemical reactions. 6. Atoms combine in one-to-one ratios to form compounds. 7. Atoms of one element are different from a ...
Review Questions 1. How many protons does potassium have? 2
... d. assumes that atoms of all elements are identical 18. The fact that lead forms two oxides of different formulas, PbO and PbO2, is an example of the a. periodic law b. law of multiple proportions c. atomic law d. law of conservation of mass 19. Water, H2O, has a mass ratio of oxygen to hydrogen of ...
... d. assumes that atoms of all elements are identical 18. The fact that lead forms two oxides of different formulas, PbO and PbO2, is an example of the a. periodic law b. law of multiple proportions c. atomic law d. law of conservation of mass 19. Water, H2O, has a mass ratio of oxygen to hydrogen of ...
Ch. 2. Atomic Structure and Periodic Table
... Nonmetals can be noticeably different from each other. Chemical Properties: They tend to share or gain electrons during chemical reactions, or not react. Boron Family (Fam. 13) Aluminum is the most abundant metal in earth’s crust, conducts electricity, and lightweight. Carbon Family (Fam. 14) ...
... Nonmetals can be noticeably different from each other. Chemical Properties: They tend to share or gain electrons during chemical reactions, or not react. Boron Family (Fam. 13) Aluminum is the most abundant metal in earth’s crust, conducts electricity, and lightweight. Carbon Family (Fam. 14) ...
Atomic Structure
... Scientist use units known as Atomic mass units (amu) A proton or a neutron has a mass equal to about 1/1000th Atomic Mass is equal to the number of protons and neutrons in an atom. ...
... Scientist use units known as Atomic mass units (amu) A proton or a neutron has a mass equal to about 1/1000th Atomic Mass is equal to the number of protons and neutrons in an atom. ...
Nuclear For Forensics
... How does one tap all that energy? Nuclear fission is the type of reaction carried out in nuclear reactors. ...
... How does one tap all that energy? Nuclear fission is the type of reaction carried out in nuclear reactors. ...
Practice problems for chapter 1, 2 and 3 1) A small amount of salt
... Practice problems for chapter 1, 2 and 3 1) A small amount of salt dissolved in water is an example of a __________. 2) Which one of the following is a pure substance? A) concrete B) wood C) salt water D) elemental copper E) milk 3) For which of the following can the composition vary? A) pure substa ...
... Practice problems for chapter 1, 2 and 3 1) A small amount of salt dissolved in water is an example of a __________. 2) Which one of the following is a pure substance? A) concrete B) wood C) salt water D) elemental copper E) milk 3) For which of the following can the composition vary? A) pure substa ...
The Atom Notes
... • Particles too small to be measured in grams/kilograms • Scientists use atomic mass units (amu) ...
... • Particles too small to be measured in grams/kilograms • Scientists use atomic mass units (amu) ...
Print › Biochemistry | Quizlet
... activation energy: minimum amount of energy needed for reactants to form products in a chemical reaction active site: specific place where a substrate binds on an enzyme amino acid: carbon compound joined by peptide bonds; building block of proteins atom: building block of matter; contains subatomic ...
... activation energy: minimum amount of energy needed for reactants to form products in a chemical reaction active site: specific place where a substrate binds on an enzyme amino acid: carbon compound joined by peptide bonds; building block of proteins atom: building block of matter; contains subatomic ...
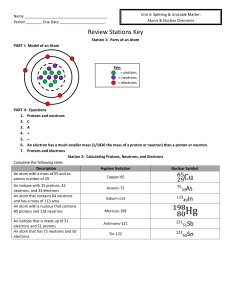
Name Period ______ Due Date Review Stations Key Station 1
... Stopped by paper, wood, cloth, etc. ...
... Stopped by paper, wood, cloth, etc. ...
Year 8 Science Assessment Point 2
... all atoms in a compound 3. Limiting reactants: A reactant that is used up in a chemical reaction and stops it from continuing 4. Chromatography: A technique where a mixture is separated For example: • Magnesium reacts with hydrochloric acid. • When the reaction is over: Magnesium is the limiting rea ...
... all atoms in a compound 3. Limiting reactants: A reactant that is used up in a chemical reaction and stops it from continuing 4. Chromatography: A technique where a mixture is separated For example: • Magnesium reacts with hydrochloric acid. • When the reaction is over: Magnesium is the limiting rea ...
electron
... mass 10.012 amu and a relative abundance of 19.91%. The isotope with mass 11.009 amu has a relative abundance of 80.09%. 1. Calculate the atomic mass of this element (show all work) and then name this element. ...
... mass 10.012 amu and a relative abundance of 19.91%. The isotope with mass 11.009 amu has a relative abundance of 80.09%. 1. Calculate the atomic mass of this element (show all work) and then name this element. ...
Isotopic labeling

Isotopic labeling (or isotopic labelling) is a technique used to track the passage of an isotope, or an atom with a variation, through a reaction, metabolic pathway, or cell. The reactant is 'labeled' by replacing specific atoms by their isotope. The reactant is then allowed to undergo the reaction. The position of the isotopes in the products is measured to determine the sequence the isotopic atom followed in the reaction or the cell's metabolic pathway. The nuclides used in isotopic labeling may be stable nuclides or radionuclides. In the latter case, the labeling is called radiolabeling.In isotopic labeling, there are multiple ways to detect the presence of labeling isotopes; through their mass, vibrational mode, or radioactive decay. Mass spectrometry detects the difference in an isotope's mass, while infrared spectroscopy detects the difference in the isotope's vibrational modes. Nuclear magnetic resonance detects atoms with different gyromagnetic ratios. The radioactive decay can be detected through an ionization chamber or autoradiographs of gels.An example of the use of isotopic labeling is the study of phenol (C6H5OH) in water by replacing common hydrogen (protium) with deuterium (deuterium labeling). Upon adding phenol to deuterated water (water containing D2O in addition to the usual H2O), the substitution of deuterium for the hydrogen is observed in phenol's hydroxyl group (resulting in C6H5OD), indicating that phenol readily undergoes hydrogen-exchange reactions with water. Only the hydroxyl group was affected, indicating that the other 5 hydrogen atoms did not participate in these exchange reactions.