Distinguishing Among Atoms
... different numbers of neutrons, they also have different mass numbers. ...
... different numbers of neutrons, they also have different mass numbers. ...
File - Biochemistry
... atoms are the smallest particles and each substance had its own type of atom - wood atoms, air atoms, water atoms Dalton: 1. all matter is made of tiny particles called atoms 2. atoms can’t be broken down further 3. atoms of different elements differ 4. atoms of the same element are identical 5. ato ...
... atoms are the smallest particles and each substance had its own type of atom - wood atoms, air atoms, water atoms Dalton: 1. all matter is made of tiny particles called atoms 2. atoms can’t be broken down further 3. atoms of different elements differ 4. atoms of the same element are identical 5. ato ...
IB496-April 10 - School of Life Sciences
... analysis (META). The main analytical engine was 13C isotopomer profiling using a combination of multi-nuclear 2-D NMR and GC-MS techniques. Using 13C-glucose as a tracer, multiple disruptions to the central metabolic network in A549 cells induced by selenite were defined. META was then achieved by c ...
... analysis (META). The main analytical engine was 13C isotopomer profiling using a combination of multi-nuclear 2-D NMR and GC-MS techniques. Using 13C-glucose as a tracer, multiple disruptions to the central metabolic network in A549 cells induced by selenite were defined. META was then achieved by c ...
Atom (A) or Ion
... 82. What is pH and how is it measured? 83. What factors affect solubility? 84. What is molarity? 85. If I have 2.5 mol of calcium carbonate in .3 L of solution, what is the molarity? 86. If I have 700 mL of a 5 M NaOH solution, how many grams of NaOH were used to make the solution? 87. What are coll ...
... 82. What is pH and how is it measured? 83. What factors affect solubility? 84. What is molarity? 85. If I have 2.5 mol of calcium carbonate in .3 L of solution, what is the molarity? 86. If I have 700 mL of a 5 M NaOH solution, how many grams of NaOH were used to make the solution? 87. What are coll ...
Atom (A) or Ion (I)
... 82. What is pH and how is it measured? 83. What factors affect solubility? 84. What is molarity? 85. If I have 2.5 mol of calcium carbonate in .3 L of solution, what is the molarity? 86. If I have 700 mL of a 5 M NaOH solution, how many grams of NaOH were used to make the solution? 87. What are coll ...
... 82. What is pH and how is it measured? 83. What factors affect solubility? 84. What is molarity? 85. If I have 2.5 mol of calcium carbonate in .3 L of solution, what is the molarity? 86. If I have 700 mL of a 5 M NaOH solution, how many grams of NaOH were used to make the solution? 87. What are coll ...
Chapter 3 Discovering the atom and subatomic particles (History of
... but has the same (reverse) electric charge as an electron. The number of protons each atom of a given element contains is called atomic number. Neutron (中子) is another subatomic particle in nucleus, having the similar mass as the proton but electrically neutral. It has very important role in holding ...
... but has the same (reverse) electric charge as an electron. The number of protons each atom of a given element contains is called atomic number. Neutron (中子) is another subatomic particle in nucleus, having the similar mass as the proton but electrically neutral. It has very important role in holding ...
Chapter 3 Discovering the atom and subatomic particles (History of
... but has the same (reverse) electric charge as an electron. The number of protons each atom of a given element contains is called atomic number. Neutron (中子) is another subatomic particle in nucleus, having the similar mass as the proton but electrically neutral. It has very important role in holding ...
... but has the same (reverse) electric charge as an electron. The number of protons each atom of a given element contains is called atomic number. Neutron (中子) is another subatomic particle in nucleus, having the similar mass as the proton but electrically neutral. It has very important role in holding ...
Structure-Prop of Matter session
... A solution is different from other types of matter like an element, compound, or mixture. A solution is a mixture of two or more substances where all parts are identical. The parts will not settle out upon standing and cannot be filtered. Yet, they are not chemically combined like in a chemical reac ...
... A solution is different from other types of matter like an element, compound, or mixture. A solution is a mixture of two or more substances where all parts are identical. The parts will not settle out upon standing and cannot be filtered. Yet, they are not chemically combined like in a chemical reac ...
Atom (A) or Ion (I)
... 82. What is pH and how is it measured? 83. What factors affect solubility? 84. What is molarity? 85. If I have 2.5 mol of calcium carbonate in .3 L of solution, what is the molarity? 86. If I have 700 mL of a 5 M NaOH solution, how many grams of NaOH were used to make the solution? 87. What are coll ...
... 82. What is pH and how is it measured? 83. What factors affect solubility? 84. What is molarity? 85. If I have 2.5 mol of calcium carbonate in .3 L of solution, what is the molarity? 86. If I have 700 mL of a 5 M NaOH solution, how many grams of NaOH were used to make the solution? 87. What are coll ...
Energy per nucleon
... • Animals and humans eat plants and incorporate 14C into the bones. • Decaying 14C is replenished as long as plants and animals are alive. • Once a plant or animal dies, its 14C content decreases and thereby starts the clock for radiocarbon dating. • By measuring the 14C/12C ratio of a sample from a ...
... • Animals and humans eat plants and incorporate 14C into the bones. • Decaying 14C is replenished as long as plants and animals are alive. • Once a plant or animal dies, its 14C content decreases and thereby starts the clock for radiocarbon dating. • By measuring the 14C/12C ratio of a sample from a ...
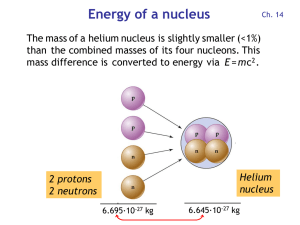
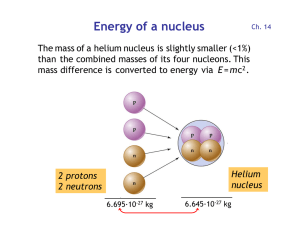
Energy of a nucleus
... • Animals and humans eat plants and incorporate 14C into the bones. • Decaying 14C is replenished as long as plants and animals are alive. • Once a plant or animal dies, its 14C content decreases and thereby starts the clock for radiocarbon dating. • By measuring the 14C/12C ratio of a sample from a ...
... • Animals and humans eat plants and incorporate 14C into the bones. • Decaying 14C is replenished as long as plants and animals are alive. • Once a plant or animal dies, its 14C content decreases and thereby starts the clock for radiocarbon dating. • By measuring the 14C/12C ratio of a sample from a ...
ExamView - ev chap 4.tst
... 5. Who conducted experiments to determine the quantity of charge carried by an electron? A. Millikan B. Thomson C. Rutherford D. Dalton 6. Which of the following was NOT among Democritus’s ideas? A. Atoms retain their identity in a chemical reaction. B. Atoms are indestructible. C. Atoms are indivis ...
... 5. Who conducted experiments to determine the quantity of charge carried by an electron? A. Millikan B. Thomson C. Rutherford D. Dalton 6. Which of the following was NOT among Democritus’s ideas? A. Atoms retain their identity in a chemical reaction. B. Atoms are indestructible. C. Atoms are indivis ...
Chapter 3 Overview - Greensburg.k12.in.us
... dehydration synthesis (condensation reaction). During dehydration synthesis, a hydroxyl (OH) group is removed from one monomer and a hydrogen is removed from the other to join them together to form a polymer. During this process, water is produced (see left). ...
... dehydration synthesis (condensation reaction). During dehydration synthesis, a hydroxyl (OH) group is removed from one monomer and a hydrogen is removed from the other to join them together to form a polymer. During this process, water is produced (see left). ...
atoms - KMKunz
... The two naturally occurring isotopes of copper are copper-63, mass 62.9298 u, and copper-65, mass 64.9278 u. What must be the percent natural abundances of the two isotopes if the atomic mass of copper listed in a table of atomic masses is 63.546 u? ...
... The two naturally occurring isotopes of copper are copper-63, mass 62.9298 u, and copper-65, mass 64.9278 u. What must be the percent natural abundances of the two isotopes if the atomic mass of copper listed in a table of atomic masses is 63.546 u? ...
Metals
... “elements”: air, fire, water, and earth. People believed this for many centuries! • In the late 1600s, early chemists began to discover that this was not the case, that there are more than 4 elements and they are not what the Greeks thought they were. • Now we know that all matter in the universe is ...
... “elements”: air, fire, water, and earth. People believed this for many centuries! • In the late 1600s, early chemists began to discover that this was not the case, that there are more than 4 elements and they are not what the Greeks thought they were. • Now we know that all matter in the universe is ...
2.4 – Exchanging gases – Further questions and
... Isotopes act like markers that illuminate and therefore identify any abnormal tissue during imaging. Usually a patient will be given a drink containing a particular kind of isotope; sometimes an injection is used depending on the target tissue. The kind of isotope that is used depends on the body ti ...
... Isotopes act like markers that illuminate and therefore identify any abnormal tissue during imaging. Usually a patient will be given a drink containing a particular kind of isotope; sometimes an injection is used depending on the target tissue. The kind of isotope that is used depends on the body ti ...
Life Substances
... What are their functions? What elements make uP Proteins? Define amino acids. How many amino acids are there? What makes one amino acid different from another? What do they look like? How are amino acids linked together? Define peptide bond What determines the kind of protein you have? Are hydrogen ...
... What are their functions? What elements make uP Proteins? Define amino acids. How many amino acids are there? What makes one amino acid different from another? What do they look like? How are amino acids linked together? Define peptide bond What determines the kind of protein you have? Are hydrogen ...
Overall Score: _____ / 22 (each question is worth
... packed together, so it does not take as much heat to give the particles enough energy to separate them. SPONCH! (Sulfur, phosphorous, oxygen, nitrogen, carbon, and hydrogen) Macromolecules are large, complicated molecules. Examples of macromolecules include DNA, protein, fat, and carbohydrates. Micr ...
... packed together, so it does not take as much heat to give the particles enough energy to separate them. SPONCH! (Sulfur, phosphorous, oxygen, nitrogen, carbon, and hydrogen) Macromolecules are large, complicated molecules. Examples of macromolecules include DNA, protein, fat, and carbohydrates. Micr ...
Chapter 4 Chemical Foundations: Elements, Atoms, and Ions
... 4. Atoms of one element can combine with atoms of other elements to form compounds. A given compound always has the same relative numbers and types of atoms. 5. Atoms are indivisible in chemical processes. That is, atoms are not created or destroyed in chemical reactions. A chemical reaction simply ...
... 4. Atoms of one element can combine with atoms of other elements to form compounds. A given compound always has the same relative numbers and types of atoms. 5. Atoms are indivisible in chemical processes. That is, atoms are not created or destroyed in chemical reactions. A chemical reaction simply ...
STURCTURES AND PROPERTIES OF MATTER
... Nucleus is the center of the atom, contains 99.9% of the mass of the atom, holds neutrons and protons. - Proton, p+: has a positive charge; all are identical no matter which element; mass is one amu; the number of protons determines which element you have – also called the atomic number. - Neutron, ...
... Nucleus is the center of the atom, contains 99.9% of the mass of the atom, holds neutrons and protons. - Proton, p+: has a positive charge; all are identical no matter which element; mass is one amu; the number of protons determines which element you have – also called the atomic number. - Neutron, ...
File
... b. How do you know? _____________________________________________________________________ c. Identify the solute: _____________________________________________________________________ d. Identify the solvent: ____________________________________________________________________ 1. List the 4 signs of ...
... b. How do you know? _____________________________________________________________________ c. Identify the solute: _____________________________________________________________________ d. Identify the solvent: ____________________________________________________________________ 1. List the 4 signs of ...
final exam review chapter 1-4
... d. ___K + ___Br2 KBr e. ___P4 + ___O2 P2O5 f. ___C7H16 + ___O2 ___CO2 + ___H2O g. ___C3H5OH + ___O2 ___CO2 + ___H2O 4. Write and balance the following reactions: a. Zinc Carbonate can be heated to form Zinc Oxide and Carbon Dioxide ...
... d. ___K + ___Br2 KBr e. ___P4 + ___O2 P2O5 f. ___C7H16 + ___O2 ___CO2 + ___H2O g. ___C3H5OH + ___O2 ___CO2 + ___H2O 4. Write and balance the following reactions: a. Zinc Carbonate can be heated to form Zinc Oxide and Carbon Dioxide ...
atomic number
... were stated by Epicurus(341-270 BC), who wrote "the sum total of things was always such as it is now, and such it will ever remain,". ...
... were stated by Epicurus(341-270 BC), who wrote "the sum total of things was always such as it is now, and such it will ever remain,". ...
File
... relate changes to individual atoms Average atom size: • Mass = 1 x 10 –23 g • Diameter = 1 x 10-8 cm How small is that?100,000,000 copper atoms in a row would = 1 cm in length! ...
... relate changes to individual atoms Average atom size: • Mass = 1 x 10 –23 g • Diameter = 1 x 10-8 cm How small is that?100,000,000 copper atoms in a row would = 1 cm in length! ...
Unit 3 Study Guide: Atomic Structure and Nuclear
... _______________ 6. Neptunium and plutonium were the first transuranium elements discovered. _______________ 7. The nuclear formula for a neutron is n. _______________ 8. The half-life of a radioisotope is the time it takes for that isotope to decay. _______________ 9. A radioisotope that decays very ...
... _______________ 6. Neptunium and plutonium were the first transuranium elements discovered. _______________ 7. The nuclear formula for a neutron is n. _______________ 8. The half-life of a radioisotope is the time it takes for that isotope to decay. _______________ 9. A radioisotope that decays very ...
Isotopic labeling

Isotopic labeling (or isotopic labelling) is a technique used to track the passage of an isotope, or an atom with a variation, through a reaction, metabolic pathway, or cell. The reactant is 'labeled' by replacing specific atoms by their isotope. The reactant is then allowed to undergo the reaction. The position of the isotopes in the products is measured to determine the sequence the isotopic atom followed in the reaction or the cell's metabolic pathway. The nuclides used in isotopic labeling may be stable nuclides or radionuclides. In the latter case, the labeling is called radiolabeling.In isotopic labeling, there are multiple ways to detect the presence of labeling isotopes; through their mass, vibrational mode, or radioactive decay. Mass spectrometry detects the difference in an isotope's mass, while infrared spectroscopy detects the difference in the isotope's vibrational modes. Nuclear magnetic resonance detects atoms with different gyromagnetic ratios. The radioactive decay can be detected through an ionization chamber or autoradiographs of gels.An example of the use of isotopic labeling is the study of phenol (C6H5OH) in water by replacing common hydrogen (protium) with deuterium (deuterium labeling). Upon adding phenol to deuterated water (water containing D2O in addition to the usual H2O), the substitution of deuterium for the hydrogen is observed in phenol's hydroxyl group (resulting in C6H5OD), indicating that phenol readily undergoes hydrogen-exchange reactions with water. Only the hydroxyl group was affected, indicating that the other 5 hydrogen atoms did not participate in these exchange reactions.