* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download atoms - KMKunz
Survey
Document related concepts
Transcript
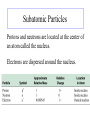
Unit 2 Atoms, Molecules, Ions, and Chemical Nomenclature Section 1 Laws of Chemical Combination and Atomic Theory Laws of Chemical Composition Conservation of Mass - The total mass remains constant during a chemical reaction. Example: Decomposition of mercuric oxide (HgO) HgO(s) = Hg(l) + O2(g) Law of Definite Proportions All samples of a compound have the same composition; that is, all samples have the same proportions, by mass, of the elements present Water always contains: ~89% oxygen ~11% hydrogen Law of Definite Proportions Basic Copper Carbonate Example 2.2 The mass ratio of oxygen to magnesium in the compound magnesium oxide is 0.6583:1. What mass of magnesium oxide will form when 2.000 g of magnesium is completely converted to magnesium oxide by burning in pure oxygen gas? Example 2.2A What mass of magnesium oxide is formed when 1.500 g of oxygen combines with magnesium? Example 2.2B When a strip of magnesium metal was burned in pure oxygen gas, 1.554 g of oxygen was consumed and the only product formed was magnesium oxide. What must have been the masses of magnesium metal burned and magnesium oxide formed? Law of Multiple Proportions When two or more different compounds of the same two elements are compared, the masses of one element that combine with the a fixed mass of the second element are in the ratio of small whole numbers. Law of Multiple Proportions • Four different oxides of nitrogen can be formed by combining 28 g of nitrogen with: • 16 g oxygen, forming Compound I • 48 g oxygen, forming Compound II • 64 g oxygen, forming Compound III • 80 g oxygen, forming Compound IV What is the ratio 16:48:64:80 expressed as small whole numbers? • Compounds I–IV are N2O, N2O3, N2O4, N2O5 Law of Multiple Proportions Dalton’s Atomic Theory Proposed in 1803 to explain the law of conservation of mass, law of definite proportions, and law of multiple proportions. • • • • • Matter is composed of atoms: tiny, indivisible particles. All atoms of a given element are the same. Atoms of one element differ from atoms of other elements. Compounds are formed when atoms of different elements unite in fixed proportions. A chemical reaction involves rearrangement of atoms. No atoms are created, destroyed, or broken apart. Dalton’s Atomic Theory • All matter is composed of extremely small, indivisible particles called atoms • All atoms of a given element are alike in mass and other properties, but atoms of one element differ from the atoms of every other element • Compounds are formed when atoms of different elements unite in fixed proportions • A chemical reaction involves a rearrangement of atoms. No atoms are created, destroyed, or broken apart in a chemical reaction Dalton’s Atomic Theory: Conservation of Mass and Definite Proportions Six fluorine atoms and four hydrogen atoms before reaction … 14 … six fluorine atoms and four hydrogen atoms after reaction. Mass is conserved. HF always has one H atom and one F atom; always has the same proportions (1:19) by mass. Section 2 Subatomic Particles, Atomic Masses, and the Periodic Table Subatomic Particles Protons and neutrons are located at the center of an atom called the nucleus. Electrons are dispersed around the nucleus. Isotopes Atoms that have the same number of protons but different numbers of neutrons are called isotopes Atomic number (Z) = number of protons Hydrogen has 1 proton, 0 neutrons Deuterium has 1 proton, 1 neutron Tritium has 1 proton, 2 neutrons - Z=1 Z=1 Z=1 Other Examples of Isotopes Carbon-14 Z=6 so 8 neutrons Chlorine-35 Z = 17 so 18 neutrons Uranium-234 Z = 92 so 142 neutrons The number of neutrons = A – Z Isotopes (cont’d) Atoms can be represented using the element’s symbol and the mass number (A) and atomic number (Z): A Z E 35 17 Cl 37 17 Cl • How many protons are in chlorine-35? • How many protons are in chlorine-37? • How many neutrons are in chlorine-37? Example 2.3 How many protons, neutrons, and electrons are present in a 81Br atom? Atomic Masses Atomic Masses An atomic mass unit (amu) is defined as exactly one-twelfth the mass of a carbon-12 atom 1 u = 1.66054 × 10–24 g The atomic mass of an element is the weighted average of the masses of the naturally occurring isotopes of that element Atomic Mass (cont’d) • Question: do all isotopes of an element have the same mass? Why or why not? • The atomic mass given on the periodic table is the weighted average of the masses of the naturally occurring isotopes of that element. 25 Example 2.4 Use the data cited above to determine the weighted average atomic mass of carbon. Example 2.4A There are three naturally occurring isotopes of neon. Their percent abundance and atomic masses are neon20, 90.51%, 19.99244 u; neon-21, 0.27%, 20.99395 u; neon-22, 9.22%, 21.99138 u. Calculate the weighted average atomic mass of neon. Example 2.4B The two naturally occurring isotopes of copper are copper-63, mass 62.9298 u, and copper-65, mass 64.9278 u. What must be the percent natural abundances of the two isotopes if the atomic mass of copper listed in a table of atomic masses is 63.546 u? Example 2.5 Indium has two naturally occurring isotopes and a weighted average atomic mass of 114.82 u. One of the isotopes has a mass of 112.9043 u. Which is likely to be the second isotope: 111In, 112In, 114In, or 115In? Example 2.5A The masses of three naturally occurring isotopes of magnesium are Mg-24, 23.98504 u; Mg-25, 24.98584 u; Mg-26, 25.98259 u. Can you determine which of the three is most abundant? Second most abundant? Mendeleev’s Periodic Table • Mendeleev arranged the known elements in order of increasing atomic weight from left to right and from top to bottom in groups. • Elements that closely resembled one another were arranged in the same vertical group. • Gaps were left where undiscovered elements should appear. • From the locations of the gaps, he was able to predict properties of some of the undiscovered elements. 31 Germanium: Prediction vs. Observation 32 Modern Periodic Table Elements are divided into two main classes EOS Chapter 2: Atoms, Molecules, and Ions 33 Modern Periodic Table Except for hydrogen, those elements to the left of the line are metals EOS Chapter 2: Atoms, Molecules, and Ions 34 Modern Periodic Table Elements to the right of the line are nonmetals EOS Chapter 2: Atoms, Molecules, and Ions 35 Modern Periodic Table Elements around the line are referred to as metalloids EOS Chapter 2: Atoms, Molecules, and Ions 36