Advanced Higher - Hodder Education
... This model paper is free to download and use for revision purposes. The paper, which may include a limited number of previously published SQA questions, has been specially commissioned by Hodder Gibson, and has been written by experienced senior teachers and examiners. This is not SQA material but ...
... This model paper is free to download and use for revision purposes. The paper, which may include a limited number of previously published SQA questions, has been specially commissioned by Hodder Gibson, and has been written by experienced senior teachers and examiners. This is not SQA material but ...
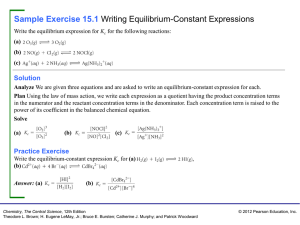
Sample Exercise 15.1 Writing Equilibrium
... carbonate and its decomposition products, calcium oxide and carbon dioxide. Plan For equilibrium to be achieved, it must be possible for both the forward process and the reverse process to occur. For the forward process to occur, there must be some calcium carbonate present. For the reverse process ...
... carbonate and its decomposition products, calcium oxide and carbon dioxide. Plan For equilibrium to be achieved, it must be possible for both the forward process and the reverse process to occur. For the forward process to occur, there must be some calcium carbonate present. For the reverse process ...
Chapter Three - CNG Chemistry
... side of the equation, try balancing that element first. • Balance any reactants or products that exist as the free element last. • In some reactions, certain groupings of atoms (such as polyatomic ions) remain unchanged. In such cases, treat these groupings as a unit. • At times, an equation can be ...
... side of the equation, try balancing that element first. • Balance any reactants or products that exist as the free element last. • In some reactions, certain groupings of atoms (such as polyatomic ions) remain unchanged. In such cases, treat these groupings as a unit. • At times, an equation can be ...
From Kinetics to Equilibrium
... When a reaction involves gases, the pressure of the system often changes as the reaction progresses. Chemists can monitor this pressure change. For example, consider the decomposition of dinitrogen pentoxide, shown in the following chemical reaction. ...
... When a reaction involves gases, the pressure of the system often changes as the reaction progresses. Chemists can monitor this pressure change. For example, consider the decomposition of dinitrogen pentoxide, shown in the following chemical reaction. ...
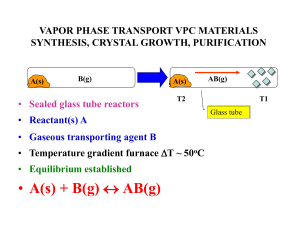
vapor phase transport vpc materials synthesis, crystal growth
... screening currents" that flow at the surface of the superconductor and which generate a magnetic field that exactly cancels (repels) the externally applied field inside the superconductor (Lenz’s law). ...
... screening currents" that flow at the surface of the superconductor and which generate a magnetic field that exactly cancels (repels) the externally applied field inside the superconductor (Lenz’s law). ...
2 - Montville.net
... world of atoms and molecules to the real world of grams . Stoichiometry problems are classified between the information given in the problem and the information you are expected to find, the unknown. The given and the unknown may be expressed in grams or moles. The masses in the reaction are usually ...
... world of atoms and molecules to the real world of grams . Stoichiometry problems are classified between the information given in the problem and the information you are expected to find, the unknown. The given and the unknown may be expressed in grams or moles. The masses in the reaction are usually ...
Changing Matter
... Step 1 Calculate the grams of AgBr that can form from each reactant. The conversion needed is g reactant → mol reactant → mol AgBr → g AgBr 1 mol MgBr2 2 mol AgBr 187.8 g AgBr 102 g AgBr ...
... Step 1 Calculate the grams of AgBr that can form from each reactant. The conversion needed is g reactant → mol reactant → mol AgBr → g AgBr 1 mol MgBr2 2 mol AgBr 187.8 g AgBr 102 g AgBr ...
text
... when its enthalpy, ∆H, decreases and its entropy, ∆S, increases. Substitut‑ ing the inequalities ∆H < 0 and ∆S > 0 into equation 6.2 shows that a reaction is thermodynamically favorable when ∆G is negative. When ∆G is positive the reaction is unfavorable as written (although the reverse reaction is ...
... when its enthalpy, ∆H, decreases and its entropy, ∆S, increases. Substitut‑ ing the inequalities ∆H < 0 and ∆S > 0 into equation 6.2 shows that a reaction is thermodynamically favorable when ∆G is negative. When ∆G is positive the reaction is unfavorable as written (although the reverse reaction is ...
Photogeneration of Hydride Donors and Their Use Toward CO2
... Our theoretical calculations predict that free CO is difficult to convert to the formyl anion by hydride transfer reactions, however, M−CO is much easier to ΔH‡ = −0.6 kcal/mol convert to M−CHO. Our calculations also show that ΔG‡ = 12.6 kcal/mol the further photoreduction of [1•HH]2+ can create a [ ...
... Our theoretical calculations predict that free CO is difficult to convert to the formyl anion by hydride transfer reactions, however, M−CO is much easier to ΔH‡ = −0.6 kcal/mol convert to M−CHO. Our calculations also show that ΔG‡ = 12.6 kcal/mol the further photoreduction of [1•HH]2+ can create a [ ...
File
... SCH4U b) Determine the oxidation number of the sulfur in the sulfate ion, SO4-2. Remember that the sum of the oxidation numbers must equal the charge of the ion. Step 1: Write All Known Oxidation Numbers Use the symbol N to represent the oxidation number of sulfur in the sulfate. ...
... SCH4U b) Determine the oxidation number of the sulfur in the sulfate ion, SO4-2. Remember that the sum of the oxidation numbers must equal the charge of the ion. Step 1: Write All Known Oxidation Numbers Use the symbol N to represent the oxidation number of sulfur in the sulfate. ...
Chemical Formulas and Chemical Compounds
... Some binary compounds are ionic, others are covalent. The types of bonding partially depend on the position of the elements in the periodic table. Label each of these claims as True or False; if False, specify the nature of the error. c. Binary compound involving metalloids are always ionic. False; ...
... Some binary compounds are ionic, others are covalent. The types of bonding partially depend on the position of the elements in the periodic table. Label each of these claims as True or False; if False, specify the nature of the error. c. Binary compound involving metalloids are always ionic. False; ...
Calculations with Chemical Formulas and Equations
... (if citrate limiting) 0.012 mol 0.012(1/3)=.0040mol 0.012 moles CO2 44g/mol(0.012mol)=0.53g CO2 .0052-.0040=.0012 left 0.0012mol(192g/mol)= Stoichiometry 0.023 g left. ...
... (if citrate limiting) 0.012 mol 0.012(1/3)=.0040mol 0.012 moles CO2 44g/mol(0.012mol)=0.53g CO2 .0052-.0040=.0012 left 0.0012mol(192g/mol)= Stoichiometry 0.023 g left. ...
2007_UG - St.Joseph`s College
... Significance of ψ and ψ2 – Radial and angular distribution function – Concept and Shapes of orbitals. Gaseous state – The gas constant R in different units - deviation from ideal behaviour – van der Waals equation for real gases – critical phenomenon – PV isotherm of real gases, critical temperature ...
... Significance of ψ and ψ2 – Radial and angular distribution function – Concept and Shapes of orbitals. Gaseous state – The gas constant R in different units - deviation from ideal behaviour – van der Waals equation for real gases – critical phenomenon – PV isotherm of real gases, critical temperature ...
CH 151 Companion
... f. Do not put pipets directly in any reagent bottle. This might result in contamination of the remaining liquid in the bottle. Never mouth pipet any liquid in the lab. g. Keep the lids and caps on the chemical bottles. Put the lids back on as soon as you are finished dispensing the material. Many ch ...
... f. Do not put pipets directly in any reagent bottle. This might result in contamination of the remaining liquid in the bottle. Never mouth pipet any liquid in the lab. g. Keep the lids and caps on the chemical bottles. Put the lids back on as soon as you are finished dispensing the material. Many ch ...
Chapter 13 Organic Chemistry
... Alkenes are organic compounds that contain carbon-carbon double bonds. The two simplest alkenes, C2H4 and C3H6, are shown in Figure 13.6. It might appear that the C3H6 molecules shown in Figures 13.6b and 13.6c are different, but they can be interchanged by a simple rotation, so they are representat ...
... Alkenes are organic compounds that contain carbon-carbon double bonds. The two simplest alkenes, C2H4 and C3H6, are shown in Figure 13.6. It might appear that the C3H6 molecules shown in Figures 13.6b and 13.6c are different, but they can be interchanged by a simple rotation, so they are representat ...
Stoichiometry
... oxygen O2 to give carbon dioxide and water. A sample of ethane was burned completely and the water that formed has a mass of 1.61 grams. How many grams of ethane was in the sample? 0.90 grams of ethane ...
... oxygen O2 to give carbon dioxide and water. A sample of ethane was burned completely and the water that formed has a mass of 1.61 grams. How many grams of ethane was in the sample? 0.90 grams of ethane ...
Module 1 Predictor Questions
... Rules for determining the number of significant figures in the answer to a calculation depend on the mathematical operation being performed. • In addition and subtraction problems, the final answer must contain no digits beyond the most doubtful digit in the numbers being added or subtracted. • In m ...
... Rules for determining the number of significant figures in the answer to a calculation depend on the mathematical operation being performed. • In addition and subtraction problems, the final answer must contain no digits beyond the most doubtful digit in the numbers being added or subtracted. • In m ...
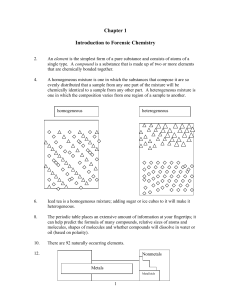
Chapter 1 Introduction to Forensic Chemistry
... oxygen (often with applied heat); carbon-containing compounds then produce carbon dioxide and hydrogen-containing compounds then produce water as a result. Neutralization reactions occur when an acid and a base react to form a salt and water. Redox reactions occur when one substance gains electrons ...
... oxygen (often with applied heat); carbon-containing compounds then produce carbon dioxide and hydrogen-containing compounds then produce water as a result. Neutralization reactions occur when an acid and a base react to form a salt and water. Redox reactions occur when one substance gains electrons ...
Tro Chemistry a Molecular Approach, 3E
... through the minute openings in your gloves (which is why my wife prefers mittens), most of it is transferred to your hands and to the pocket of air surrounding your hands, resulting in a temperature increase. The magnitude of the temperature increase depends on the size of the hand warmer and the si ...
... through the minute openings in your gloves (which is why my wife prefers mittens), most of it is transferred to your hands and to the pocket of air surrounding your hands, resulting in a temperature increase. The magnitude of the temperature increase depends on the size of the hand warmer and the si ...
Bioorthogonal chemistry
The term bioorthogonal chemistry refers to any chemical reaction that can occur inside of living systems without interfering with native biochemical processes. The term was coined by Carolyn R. Bertozzi in 2003. Since its introduction, the concept of the bioorthogonal reaction has enabled the study of biomolecules such as glycans, proteins, and lipids in real time in living systems without cellular toxicity. A number of chemical ligation strategies have been developed that fulfill the requirements of bioorthogonality, including the 1,3-dipolar cycloaddition between azides and cyclooctynes (also termed copper-free click chemistry), between nitrones and cyclooctynes, oxime/hydrazone formation from aldehydes and ketones, the tetrazine ligation, the isocyanide-based click reaction, and most recently, the quadricyclane ligation.The use of bioorthogonal chemistry typically proceeds in two steps. First, a cellular substrate is modified with a bioorthogonal functional group (chemical reporter) and introduced to the cell; substrates include metabolites, enzyme inhibitors, etc. The chemical reporter must not alter the structure of the substrate dramatically to avoid affecting its bioactivity. Secondly, a probe containing the complementary functional group is introduced to react and label the substrate.Although effective bioorthogonal reactions such as copper-free click chemistry have been developed, development of new reactions continues to generate orthogonal methods for labeling to allow multiple methods of labeling to be used in the same biosystems.