Topic 1: Quantitative chemistry (12
... TOK: The early discoverers of the elements allowed chemistry to make great steps with limited apparatus, often derived from the pseudoscience of alchemy. Lavoisier’s work with oxygen, which overturned the phlogiston theory of heat, could be discussed as an example of a paradigm shift. Int: The disco ...
... TOK: The early discoverers of the elements allowed chemistry to make great steps with limited apparatus, often derived from the pseudoscience of alchemy. Lavoisier’s work with oxygen, which overturned the phlogiston theory of heat, could be discussed as an example of a paradigm shift. Int: The disco ...
Topic 1: Quantitative chemistry (12
... TOK: The early discoverers of the elements allowed chemistry to make great steps with limited apparatus, often derived from the pseudoscience of alchemy. Lavoisier’s work with oxygen, which overturned the phlogiston theory of heat, could be discussed as an example of a paradigm shift. Int: The disco ...
... TOK: The early discoverers of the elements allowed chemistry to make great steps with limited apparatus, often derived from the pseudoscience of alchemy. Lavoisier’s work with oxygen, which overturned the phlogiston theory of heat, could be discussed as an example of a paradigm shift. Int: The disco ...
Drug stability - 성균관대학교 약학대학 물리약학 연구실
... ▪ Ex) Addition of caffeine into solution of benzocaine, procaine and tetracaine SKKU Physical Pharmacy Laboratory 성균관대학교 물리약학연구실 ...
... ▪ Ex) Addition of caffeine into solution of benzocaine, procaine and tetracaine SKKU Physical Pharmacy Laboratory 성균관대학교 물리약학연구실 ...
b - Gordon State College
... 1 mol CH4 requires 2 mol O2, available O2 is 1 mol: limiting reagent. Result: 1 mol O2 will be consumed completely and CH4 will have leftover: excess reagent. ...
... 1 mol CH4 requires 2 mol O2, available O2 is 1 mol: limiting reagent. Result: 1 mol O2 will be consumed completely and CH4 will have leftover: excess reagent. ...
Practice Problems in Biomedical Organic Chemistry
... that students might encounter during their professional careers. Our plan is to release sets of organic chemistry problems in a three volume series. The problems are intended to serve as self-directed learning tools, which students can use at a pace that meets their individual educational needs. We ...
... that students might encounter during their professional careers. Our plan is to release sets of organic chemistry problems in a three volume series. The problems are intended to serve as self-directed learning tools, which students can use at a pace that meets their individual educational needs. We ...
2014 Exams
... then reacted with H2SO4, giving a white precipitate. The decantate from this precipitate is then reacted with KCN, followed by (NH4)2S (aq), which results in a yellow solid. Decantate “A” is treated with concentrated HCl, which first produces an orange precipitate, then with more HCl the solid compl ...
... then reacted with H2SO4, giving a white precipitate. The decantate from this precipitate is then reacted with KCN, followed by (NH4)2S (aq), which results in a yellow solid. Decantate “A” is treated with concentrated HCl, which first produces an orange precipitate, then with more HCl the solid compl ...
Exam
... 34) A student had 2.0 L of a sodium hydroxide solution that had a concentration of 0.4000 M. The student needed to make 500 mL of a 0.1000 M solution. How many mL of the concentrated solution was needed? 35) A student had 5.0 L of a sulfuric acid solution available, that had a concentration of 1.000 ...
... 34) A student had 2.0 L of a sodium hydroxide solution that had a concentration of 0.4000 M. The student needed to make 500 mL of a 0.1000 M solution. How many mL of the concentrated solution was needed? 35) A student had 5.0 L of a sulfuric acid solution available, that had a concentration of 1.000 ...
advanced placement chemistry workbook and note set
... element name plus its mass number. The name of the isotope on the far left in Figure 4 is carbon-12, the middle isotope is carbon-13 and the isotope on the right is carbon-14. This system of naming isotopes helps distinguish between the varying Figure 4. You can see in the figure above that there ar ...
... element name plus its mass number. The name of the isotope on the far left in Figure 4 is carbon-12, the middle isotope is carbon-13 and the isotope on the right is carbon-14. This system of naming isotopes helps distinguish between the varying Figure 4. You can see in the figure above that there ar ...
A Few Things You Might Want To Know
... If two elements combine with each other in more than one ratio, the relative masses of the varying element form simple, whole-number ratios. ...
... If two elements combine with each other in more than one ratio, the relative masses of the varying element form simple, whole-number ratios. ...
Unit 1 Mole and enthalpy changes
... Chemical Energy Thermochemistry is the study of heat energy taken in or given out in chemical reactions. This heat, absorbed or released, can be related to the internal energy of the substances involved. Such internal energy is called ENTHALPY, symbol H. As it is only possible to measure the change ...
... Chemical Energy Thermochemistry is the study of heat energy taken in or given out in chemical reactions. This heat, absorbed or released, can be related to the internal energy of the substances involved. Such internal energy is called ENTHALPY, symbol H. As it is only possible to measure the change ...
WJEC Eduqas A Level Chemistry specification
... included in the overview will not be directly assessed. Practical work is an intrinsic part of this specification. It is vitally important in developing a conceptual understanding of many topics and it enhances the experience and enjoyment of chemistry. The practical skills developed are also fundam ...
... included in the overview will not be directly assessed. Practical work is an intrinsic part of this specification. It is vitally important in developing a conceptual understanding of many topics and it enhances the experience and enjoyment of chemistry. The practical skills developed are also fundam ...
UNITS OF CONCENTRATION
... takes into account the actual number of reacting species per mole of reagent (i.e., protons in the case of acid/base reactions or electrons in the case of redox reactions). For acids, an equivalent is defined as one mole of protons. The equivalent amount of any acid is the amount of acid that delive ...
... takes into account the actual number of reacting species per mole of reagent (i.e., protons in the case of acid/base reactions or electrons in the case of redox reactions). For acids, an equivalent is defined as one mole of protons. The equivalent amount of any acid is the amount of acid that delive ...
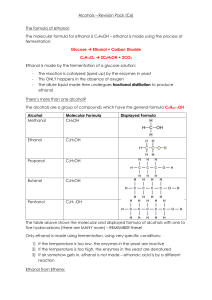
1. (a) Propan-1ol, C2H5CH2OH can be oxidised to propanoic acid
... Write the chemical equations that are relevant to the functioning of the mixture as a buffer, and use them to explain how buffering action arises. You may represent sorbic acid as RCOOH and potassium sorbate as RCOOK. ...
... Write the chemical equations that are relevant to the functioning of the mixture as a buffer, and use them to explain how buffering action arises. You may represent sorbic acid as RCOOH and potassium sorbate as RCOOK. ...
C:\SUBJECTS\SUBJECTS\Chemistry
... A hydrocarbon. Which of the following statements is FALSE? A. copper (11) ion can be reduced to copper (1) ion by hydrochloric acid and zinc. B. Sodium metal dissolves in water giving oxygen C. Nitrogen is insoluble in water D. Carbondioxide is soluble in water E. Lead has a higher atomic weight tha ...
... A hydrocarbon. Which of the following statements is FALSE? A. copper (11) ion can be reduced to copper (1) ion by hydrochloric acid and zinc. B. Sodium metal dissolves in water giving oxygen C. Nitrogen is insoluble in water D. Carbondioxide is soluble in water E. Lead has a higher atomic weight tha ...
C6 Revision Guide - West Derby School
... When a soap flake is shaken in a water sample, calcium ions in the water (from the calcium hydrogencarbonate) react with the soap to form a nasty scum. As you add more flakes, over time and after you’ve continually shaken the mixture, the soap reacts with ALL of the calcium ions. After this point, a ...
... When a soap flake is shaken in a water sample, calcium ions in the water (from the calcium hydrogencarbonate) react with the soap to form a nasty scum. As you add more flakes, over time and after you’ve continually shaken the mixture, the soap reacts with ALL of the calcium ions. After this point, a ...
Stoichiometry Objectives
... To convert between moles and mass, you need to use the atomic mass found on the periodic table. Calculate the mass of 0.625 moles of calcium. -According to the periodic table, the atomic mass of calcium is 40.078 amu, so the molar mass of calcium is 40.078 g/mol. ...
... To convert between moles and mass, you need to use the atomic mass found on the periodic table. Calculate the mass of 0.625 moles of calcium. -According to the periodic table, the atomic mass of calcium is 40.078 amu, so the molar mass of calcium is 40.078 g/mol. ...
Here`s - Sonlight
... want to measure something short, we use the inch unit, which is equal to one-twelfth of a foot. On the other hand, if we want to measure something with small volume, we might use the quart unit, which is equal to one-fourth of a gallon. In the English system, every alternative unit has a different r ...
... want to measure something short, we use the inch unit, which is equal to one-twelfth of a foot. On the other hand, if we want to measure something with small volume, we might use the quart unit, which is equal to one-fourth of a gallon. In the English system, every alternative unit has a different r ...
Bioorthogonal chemistry
The term bioorthogonal chemistry refers to any chemical reaction that can occur inside of living systems without interfering with native biochemical processes. The term was coined by Carolyn R. Bertozzi in 2003. Since its introduction, the concept of the bioorthogonal reaction has enabled the study of biomolecules such as glycans, proteins, and lipids in real time in living systems without cellular toxicity. A number of chemical ligation strategies have been developed that fulfill the requirements of bioorthogonality, including the 1,3-dipolar cycloaddition between azides and cyclooctynes (also termed copper-free click chemistry), between nitrones and cyclooctynes, oxime/hydrazone formation from aldehydes and ketones, the tetrazine ligation, the isocyanide-based click reaction, and most recently, the quadricyclane ligation.The use of bioorthogonal chemistry typically proceeds in two steps. First, a cellular substrate is modified with a bioorthogonal functional group (chemical reporter) and introduced to the cell; substrates include metabolites, enzyme inhibitors, etc. The chemical reporter must not alter the structure of the substrate dramatically to avoid affecting its bioactivity. Secondly, a probe containing the complementary functional group is introduced to react and label the substrate.Although effective bioorthogonal reactions such as copper-free click chemistry have been developed, development of new reactions continues to generate orthogonal methods for labeling to allow multiple methods of labeling to be used in the same biosystems.