03_Worked_Examples
... Sample Exercise 3.1 Interpreting and Balancing Chemical Equations The following diagram represents a chemical reaction in which the red spheres are oxygen atoms and the blue spheres are nitrogen atoms. (a) Write the chemical formulas for the reactants and products. (b) Write a balanced equation for ...
... Sample Exercise 3.1 Interpreting and Balancing Chemical Equations The following diagram represents a chemical reaction in which the red spheres are oxygen atoms and the blue spheres are nitrogen atoms. (a) Write the chemical formulas for the reactants and products. (b) Write a balanced equation for ...
03_Worked_Examples
... Sample Exercise 3.1 Interpreting and Balancing Chemical Equations The following diagram represents a chemical reaction in which the red spheres are oxygen atoms and the blue spheres are nitrogen atoms. (a) Write the chemical formulas for the reactants and products. (b) Write a balanced equation for ...
... Sample Exercise 3.1 Interpreting and Balancing Chemical Equations The following diagram represents a chemical reaction in which the red spheres are oxygen atoms and the blue spheres are nitrogen atoms. (a) Write the chemical formulas for the reactants and products. (b) Write a balanced equation for ...
Mole Concept - Shailendra Kumar Chemistry
... One of the earliest method for determining the molecular weight of protein was based on chemical analysis. A haemoglobin preparation was found to contain 0.335% iron. (a) If the haemoglobin molecule contain one atom of iron. What is its molecular weight ? (b) If the haemoglobin molecule contains fou ...
... One of the earliest method for determining the molecular weight of protein was based on chemical analysis. A haemoglobin preparation was found to contain 0.335% iron. (a) If the haemoglobin molecule contain one atom of iron. What is its molecular weight ? (b) If the haemoglobin molecule contains fou ...
Chapter 8 PowerPoint - Southeast Online
... • The equation 3 H2(g) + N2(g) 2 NH3(g) tells us that 3 molecules of H2 react with exactly 1 molecule of N2 and make exactly 2 molecules of NH3 or: 3 molecules H2 1 molecule N2 2 molecules NH3 • Since we count molecules by moles: 3 moles H2 1 mole N2 2 moles NH3 Tro's “Introductory Chemist ...
... • The equation 3 H2(g) + N2(g) 2 NH3(g) tells us that 3 molecules of H2 react with exactly 1 molecule of N2 and make exactly 2 molecules of NH3 or: 3 molecules H2 1 molecule N2 2 molecules NH3 • Since we count molecules by moles: 3 moles H2 1 mole N2 2 moles NH3 Tro's “Introductory Chemist ...
(III) ion and a cobalt (II) - Iowa State University Digital Repository
... X 10-4 M, [t-BuGOH] = 8.80 X 10"^ M, 2 cm cell) ...
... X 10-4 M, [t-BuGOH] = 8.80 X 10"^ M, 2 cm cell) ...
Chapter 5 Principles of Chemical Reactivity: Energy and Chemical
... (d) State function—Any parameter which is dependent ONLY on the initial and final states. Chemists typically use CAPITAL letters to indicate state functions (e.g. H, S, ) while nonstate functions are indicated with LOWER CASE letters (e.g. q, w). Your checking account balance is a state function! (e ...
... (d) State function—Any parameter which is dependent ONLY on the initial and final states. Chemists typically use CAPITAL letters to indicate state functions (e.g. H, S, ) while nonstate functions are indicated with LOWER CASE letters (e.g. q, w). Your checking account balance is a state function! (e ...
Equilibrium
... The value of the equilibrium constant for any reaction can be determined by experiment. As detailed in the above section, the equilibrium position for a given reaction does not depend on the starting concentrations, so the equilibrium constant has the same value regardless of the initial amounts of ...
... The value of the equilibrium constant for any reaction can be determined by experiment. As detailed in the above section, the equilibrium position for a given reaction does not depend on the starting concentrations, so the equilibrium constant has the same value regardless of the initial amounts of ...
OCR A Level Chemistry A H432 Specification
... interesting to teach and administer within all centres (large and small). Our new A Level in Chemistry A builds on our existing popular course. We’ve based the redevelopment of our A level sciences on an understanding of what works well in centres large and small and have updated areas of content an ...
... interesting to teach and administer within all centres (large and small). Our new A Level in Chemistry A builds on our existing popular course. We’ve based the redevelopment of our A level sciences on an understanding of what works well in centres large and small and have updated areas of content an ...
Ghw#8-chapter-17-Tro-F-16
... 1. If DH is negative it helps product to be favored 2. If DS is positive it helps product to be favored 3. If DG is negative reaction is product favored Gibbs free energy change = difference between the enthalpy of a system and the product of its absolute temperature and entropy predictor of spontan ...
... 1. If DH is negative it helps product to be favored 2. If DS is positive it helps product to be favored 3. If DG is negative reaction is product favored Gibbs free energy change = difference between the enthalpy of a system and the product of its absolute temperature and entropy predictor of spontan ...
Atmospheric Formation_TELTEK
... the particulate and gas phases in areas with 100 μg m-3 background aerosol.1 The dissolution of organics into rain and cloud droplets is determined by the Henry´s Law constant – this will be discussed elsewhere. Reaction with OH radicals is the dominant loss process for the majority of the troposphe ...
... the particulate and gas phases in areas with 100 μg m-3 background aerosol.1 The dissolution of organics into rain and cloud droplets is determined by the Henry´s Law constant – this will be discussed elsewhere. Reaction with OH radicals is the dominant loss process for the majority of the troposphe ...
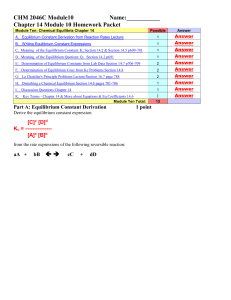
CHEM 1212 Module Ten-Chapter 16 Name
... 4. Your textbook introduces the concept of equilibrium by noting that no reaction goes fully to completion. What does this imply about the reverse reaction? ...
... 4. Your textbook introduces the concept of equilibrium by noting that no reaction goes fully to completion. What does this imply about the reverse reaction? ...
Lectures on Chapter 4, Part 2 Powerpoint 97 Document
... SO42-(aq) + 2 e Add water to the reactant side to supply an oxygen and add two protons to the product side that will remain plus the two electrons. SO32-(aq) + H2O(l) SO42-(aq) + 2 H+(aq) + 2 e Reduction: MnO4-(aq) + 3 eMnO2 (s) Add water to the product side to take up the extra oxygen from Mn cpds, ...
... SO42-(aq) + 2 e Add water to the reactant side to supply an oxygen and add two protons to the product side that will remain plus the two electrons. SO32-(aq) + H2O(l) SO42-(aq) + 2 H+(aq) + 2 e Reduction: MnO4-(aq) + 3 eMnO2 (s) Add water to the product side to take up the extra oxygen from Mn cpds, ...
AVOGADRO EXAMS 1991 - 2002 PRACTICE BOOKLET
... 16. An element occurring in nature as a metal(such as copper or gold) is likely to (a) react readily with oxygen to from a protective oxide coating (b) be at the high end of the activity series of metals (c) cause strong acids to release hydrogen gas (d) undergo oxidation only with difficulty (e) lo ...
... 16. An element occurring in nature as a metal(such as copper or gold) is likely to (a) react readily with oxygen to from a protective oxide coating (b) be at the high end of the activity series of metals (c) cause strong acids to release hydrogen gas (d) undergo oxidation only with difficulty (e) lo ...
Example of Lab Notebook
... using a Buchner funnel via vacuum filtration using cold water as a rinse. Following the reaction and allowing to air dry, the mass of the acetanilide was determined to be 5.15 g. The limiting reactant was determined to be aniline and 7.7 g of product was theoretically possible. This allowed for a lo ...
... using a Buchner funnel via vacuum filtration using cold water as a rinse. Following the reaction and allowing to air dry, the mass of the acetanilide was determined to be 5.15 g. The limiting reactant was determined to be aniline and 7.7 g of product was theoretically possible. This allowed for a lo ...
Mole Concept
... •NOTE: Skip this step if you have already identified the limiting reagent. To determine which reagent is limiting, use the mole ratio obtained from the balanced equation for the reaction to find the moles of reactant B needed to react with the available moles of reactant A. If the moles of B availab ...
... •NOTE: Skip this step if you have already identified the limiting reagent. To determine which reagent is limiting, use the mole ratio obtained from the balanced equation for the reaction to find the moles of reactant B needed to react with the available moles of reactant A. If the moles of B availab ...
Equilib - C.R.C.T.
... The Equilib module – Regular Features • Equilib calculates the conditions for multiphase, multicomponent equilibria, with a wide variety of tabular and graphical output modes, under a large range of constraints. • Equilib accesses both compound and solution databases. Table of contents ...
... The Equilib module – Regular Features • Equilib calculates the conditions for multiphase, multicomponent equilibria, with a wide variety of tabular and graphical output modes, under a large range of constraints. • Equilib accesses both compound and solution databases. Table of contents ...
Stoichiometry: Calculations with Chemical Formulas and Equations
... How much is left of the excess reagent ?. ...
... How much is left of the excess reagent ?. ...
Semester 4 - Vaal University of Technology
... The attached logbook makes it easy to keep a permanent record of all your activities during your work integrated learning period. Your tutor/mentor must certify that you satisfactorily performed the work reported. In the event of a change of employer during this period, have your logbook brought up ...
... The attached logbook makes it easy to keep a permanent record of all your activities during your work integrated learning period. Your tutor/mentor must certify that you satisfactorily performed the work reported. In the event of a change of employer during this period, have your logbook brought up ...
Chemistry Honours - SCS Autonomous College
... Bohr’s theory, its limitations and atomic spectrum of hydrogen atom. Wave mechanics: de Broglie equation, Heisenberg’s Uncertainty Principle and its significance, Schrödinger’s wave equation, significance of ψ and ψ 2 . Quantum numbers and their significance. Normalized and orthogonal wave functions ...
... Bohr’s theory, its limitations and atomic spectrum of hydrogen atom. Wave mechanics: de Broglie equation, Heisenberg’s Uncertainty Principle and its significance, Schrödinger’s wave equation, significance of ψ and ψ 2 . Quantum numbers and their significance. Normalized and orthogonal wave functions ...
Experiment 7 - MASSIVE REACTIONS
... carefully point out important aspects of the reaction that students often miss or fail to consider when working on their own. Proper investment of the teacher’s time can make all the difference in understanding chemical reactions. On the other hand, nothing can beat having the students perform the e ...
... carefully point out important aspects of the reaction that students often miss or fail to consider when working on their own. Proper investment of the teacher’s time can make all the difference in understanding chemical reactions. On the other hand, nothing can beat having the students perform the e ...
Bioorthogonal chemistry
The term bioorthogonal chemistry refers to any chemical reaction that can occur inside of living systems without interfering with native biochemical processes. The term was coined by Carolyn R. Bertozzi in 2003. Since its introduction, the concept of the bioorthogonal reaction has enabled the study of biomolecules such as glycans, proteins, and lipids in real time in living systems without cellular toxicity. A number of chemical ligation strategies have been developed that fulfill the requirements of bioorthogonality, including the 1,3-dipolar cycloaddition between azides and cyclooctynes (also termed copper-free click chemistry), between nitrones and cyclooctynes, oxime/hydrazone formation from aldehydes and ketones, the tetrazine ligation, the isocyanide-based click reaction, and most recently, the quadricyclane ligation.The use of bioorthogonal chemistry typically proceeds in two steps. First, a cellular substrate is modified with a bioorthogonal functional group (chemical reporter) and introduced to the cell; substrates include metabolites, enzyme inhibitors, etc. The chemical reporter must not alter the structure of the substrate dramatically to avoid affecting its bioactivity. Secondly, a probe containing the complementary functional group is introduced to react and label the substrate.Although effective bioorthogonal reactions such as copper-free click chemistry have been developed, development of new reactions continues to generate orthogonal methods for labeling to allow multiple methods of labeling to be used in the same biosystems.