Sustainable Oxidation Catalysis for Synthesis
... problematic on a larger scale. There is a need to develop efficient catalysts that use sustainable terminal oxidants such as molecular oxygen or hydrogen peroxide. Although such methods are employed in the preparation of commodity chemicals, they are rarely used for the synthesis of fine chemicals, ...
... problematic on a larger scale. There is a need to develop efficient catalysts that use sustainable terminal oxidants such as molecular oxygen or hydrogen peroxide. Although such methods are employed in the preparation of commodity chemicals, they are rarely used for the synthesis of fine chemicals, ...
Chapter 23 + Practice Problems - Bloomsburg Area School District
... Monosaccharides A monosaccharide is a simple sugar that is the basic subunit of a carbohydrate. A single monosaccharide molecule contains three to seven carbon atoms. Monosaccharide compounds are typically sweet-tasting, white solids at room temperature. Because they have polar, hydroxyl (IOH) group ...
... Monosaccharides A monosaccharide is a simple sugar that is the basic subunit of a carbohydrate. A single monosaccharide molecule contains three to seven carbon atoms. Monosaccharide compounds are typically sweet-tasting, white solids at room temperature. Because they have polar, hydroxyl (IOH) group ...
Review Unit: Chemistry Review
... possible. Science would not progress very far without the increasingly advanced technologies available to scientists. Often scientific advances have to wait on the development of technologies for research to be done; for example, glassware, the battery, the laser, and the computer. Often science is ...
... possible. Science would not progress very far without the increasingly advanced technologies available to scientists. Often scientific advances have to wait on the development of technologies for research to be done; for example, glassware, the battery, the laser, and the computer. Often science is ...
EDEXCEL A LeveL - Hodder Education
... 2 What steps should the students take to ensure that all the b) How much copper, in moles, combines with one mole of copper oxide is reduced to copper? oxygen in red copper oxide? 3 Start a spreadsheet program on a computer and open up c) What is the formula of red copper oxide? a new spreadsheet ...
... 2 What steps should the students take to ensure that all the b) How much copper, in moles, combines with one mole of copper oxide is reduced to copper? oxygen in red copper oxide? 3 Start a spreadsheet program on a computer and open up c) What is the formula of red copper oxide? a new spreadsheet ...
Rhenium- and molybdenum-catalyzed dehydration reactions
... dehydration reaction. In this thesis, rhenium- and molybdenum-based complexes are applied as catalysts for the dehydration reaction. Chapter 1 of this thesis provides an overview of the state of the art for the use of various organometallic rhenium complexes as catalyst for the deoxygenation of biom ...
... dehydration reaction. In this thesis, rhenium- and molybdenum-based complexes are applied as catalysts for the dehydration reaction. Chapter 1 of this thesis provides an overview of the state of the art for the use of various organometallic rhenium complexes as catalyst for the deoxygenation of biom ...
Questions
... These compounds can also be distinguished from one another by the use of concentrated sulphuric acid. (i) ...
... These compounds can also be distinguished from one another by the use of concentrated sulphuric acid. (i) ...
1b-Redox FIB notes and practice
... On the other hand, copper is ______________ in this reaction from Cu 0 to Cu +2. These results agree with those obtained by analyzing the reaction by using electron transfer. Example: Use the change in oxidation number to identify which elements are oxidized and reduced in each of these reactions. ...
... On the other hand, copper is ______________ in this reaction from Cu 0 to Cu +2. These results agree with those obtained by analyzing the reaction by using electron transfer. Example: Use the change in oxidation number to identify which elements are oxidized and reduced in each of these reactions. ...
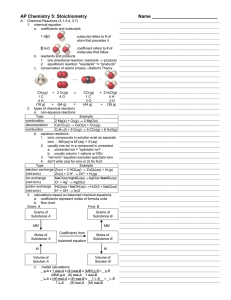
Stoichiometry - HCC Learning Web
... • A formula weight is the sum of the atomic weights for the atoms in a chemical formula. • This is the quantitative significance of a formula. • The formula weight of calcium chloride, CaCl2, would be Ca: 1(40.08 amu) + Cl: 2(35.453 amu) 110.99 amu Stoichiometry © 2015 Pearson Education, Inc. ...
... • A formula weight is the sum of the atomic weights for the atoms in a chemical formula. • This is the quantitative significance of a formula. • The formula weight of calcium chloride, CaCl2, would be Ca: 1(40.08 amu) + Cl: 2(35.453 amu) 110.99 amu Stoichiometry © 2015 Pearson Education, Inc. ...
ChemQuest 1 Information: Qualitative vs. Quantitative Critical
... 4. How are elements different from compounds? Elements are composed of only one type of atom, but compounds are composed of more than one. 5. How are compounds different from mixtures? Compounds are formed by a chemical change (i.e. two hydrogen and one oxygen atom bonding to form a water molecule), ...
... 4. How are elements different from compounds? Elements are composed of only one type of atom, but compounds are composed of more than one. 5. How are compounds different from mixtures? Compounds are formed by a chemical change (i.e. two hydrogen and one oxygen atom bonding to form a water molecule), ...
Chem12 SM Unit 5 Review final ok
... (d) In Cu(NO3)2, the oxidation number of O is –2, the oxidation number of Cu is +2, and the oxidation number of N is +5. (e) In KMnO4, the oxidation number of K is +1, the oxidation number of O is –2, and the oxidation number of Mn is +7. (f) In Na3Fe(OH)6, the oxidation number of Na is +1, the oxid ...
... (d) In Cu(NO3)2, the oxidation number of O is –2, the oxidation number of Cu is +2, and the oxidation number of N is +5. (e) In KMnO4, the oxidation number of K is +1, the oxidation number of O is –2, and the oxidation number of Mn is +7. (f) In Na3Fe(OH)6, the oxidation number of Na is +1, the oxid ...
A flask contains 0
... Look for the word approximate in the question, if there, you can use estimation to help arrive at the answer. Strategies on the multiple choice questions: All multiple choice questions count the same amount…whether it took you 5 minutes or 15 seconds. Go through the test completely once, answe ...
... Look for the word approximate in the question, if there, you can use estimation to help arrive at the answer. Strategies on the multiple choice questions: All multiple choice questions count the same amount…whether it took you 5 minutes or 15 seconds. Go through the test completely once, answe ...
SAMPLE EXAMINATION IV Section I – Multiple Choice
... In Chapter 5, we discussed reactions that go to completion; that is, reactions in which the limiting reactant is consumed and a maximum quantity of product is formed. However, in actual practice, many reaction systems reach a condition in which some quantity of each reactant remains in contact with ...
... In Chapter 5, we discussed reactions that go to completion; that is, reactions in which the limiting reactant is consumed and a maximum quantity of product is formed. However, in actual practice, many reaction systems reach a condition in which some quantity of each reactant remains in contact with ...
Unit 4 Chemical Kinetics and Chemical Equilibrium
... the concentrations into the expression for Kc. You should get the same (or very close to) the value given for Kc. ...
... the concentrations into the expression for Kc. You should get the same (or very close to) the value given for Kc. ...
3.Redox
... b. The end point is signaled by using an indicator. This is a substance that will react with one of the reactants, usually the titrant, to produce a change in color. When the solution changes color, the end point has been reached. 2. The titration reaction can be any of the solution reactions discus ...
... b. The end point is signaled by using an indicator. This is a substance that will react with one of the reactants, usually the titrant, to produce a change in color. When the solution changes color, the end point has been reached. 2. The titration reaction can be any of the solution reactions discus ...
Chapter 5 Atomic Structure
... 1. Calculation of the molar masses of atoms, ions, molecules and formula units. 2. Solutions of problems involving the relationships between the number of particles, the amount of substance in moles and the mass in grams. 3. Inter conversion of the percentage composition by mass and the empirical fo ...
... 1. Calculation of the molar masses of atoms, ions, molecules and formula units. 2. Solutions of problems involving the relationships between the number of particles, the amount of substance in moles and the mass in grams. 3. Inter conversion of the percentage composition by mass and the empirical fo ...
here
... their atoms. Since those atoms are indivisible, they obviously cannot be destroyed, so the total number of atoms in the entire system must stay the same. If the total number of atoms stays the same, the total amount of matter stays the same, and therefore, the mass stays the same. Now let’s consider ...
... their atoms. Since those atoms are indivisible, they obviously cannot be destroyed, so the total number of atoms in the entire system must stay the same. If the total number of atoms stays the same, the total amount of matter stays the same, and therefore, the mass stays the same. Now let’s consider ...
Chemistry XII - Kendriya Vidyalaya IIM,Lucknow
... O2(g) + 2H+(aq) + 4e● Can you store copper sulphate in zinc pot ? Ans: No , because Zn is more reactive than Cu . ●If a current of 0.5 ampere flows through a metallic wire for 2 hours , then how many electrons flow through the wire ? Ans : Q = it = 5 Х 2 Х 60 Х 60 = 3600 C No. of electrons flow = 36 ...
... O2(g) + 2H+(aq) + 4e● Can you store copper sulphate in zinc pot ? Ans: No , because Zn is more reactive than Cu . ●If a current of 0.5 ampere flows through a metallic wire for 2 hours , then how many electrons flow through the wire ? Ans : Q = it = 5 Х 2 Х 60 Х 60 = 3600 C No. of electrons flow = 36 ...
CHOICE BASED CREDIT SYSTEM B. Sc. WITH CHEMISTRY
... ionization, ionization constant and ionic product of water. Ionization of weak acids and bases, pH scale, common ion effect. Salt hydrolysis-calculation of hydrolysis constant, degree of hydrolysis and pH for different salts. Buffer solutions. Solubility and solubility product of sparingly soluble s ...
... ionization, ionization constant and ionic product of water. Ionization of weak acids and bases, pH scale, common ion effect. Salt hydrolysis-calculation of hydrolysis constant, degree of hydrolysis and pH for different salts. Buffer solutions. Solubility and solubility product of sparingly soluble s ...
CHAPTER 19
... either atom has totally lost or totally gained any electrons. In the case of the formation of hydrogen chloride, for example, hydrogen simply has donated a share of its bonding electron to the chlorine; it has not completely transferred that electron. The assignment of oxidation numbers allows an ap ...
... either atom has totally lost or totally gained any electrons. In the case of the formation of hydrogen chloride, for example, hydrogen simply has donated a share of its bonding electron to the chlorine; it has not completely transferred that electron. The assignment of oxidation numbers allows an ap ...
Print this article - Bangladesh Journals Online
... assignable for protons Hd and Ha respectively. The two doublets of doublet at δ 6.5 (JHa-Hb = JHb-Hc = J = 8.0 Hz) and 6.9 (JHb-Hc= JHc-Hd = J = 8.0 Hz) accounts for the Ha and Hd respectively, while the relatively downfield signal at δ 8.5 has been assigned for the imine (=N-H) proton of 2-mercapto ...
... assignable for protons Hd and Ha respectively. The two doublets of doublet at δ 6.5 (JHa-Hb = JHb-Hc = J = 8.0 Hz) and 6.9 (JHb-Hc= JHc-Hd = J = 8.0 Hz) accounts for the Ha and Hd respectively, while the relatively downfield signal at δ 8.5 has been assigned for the imine (=N-H) proton of 2-mercapto ...
Bioorthogonal chemistry
The term bioorthogonal chemistry refers to any chemical reaction that can occur inside of living systems without interfering with native biochemical processes. The term was coined by Carolyn R. Bertozzi in 2003. Since its introduction, the concept of the bioorthogonal reaction has enabled the study of biomolecules such as glycans, proteins, and lipids in real time in living systems without cellular toxicity. A number of chemical ligation strategies have been developed that fulfill the requirements of bioorthogonality, including the 1,3-dipolar cycloaddition between azides and cyclooctynes (also termed copper-free click chemistry), between nitrones and cyclooctynes, oxime/hydrazone formation from aldehydes and ketones, the tetrazine ligation, the isocyanide-based click reaction, and most recently, the quadricyclane ligation.The use of bioorthogonal chemistry typically proceeds in two steps. First, a cellular substrate is modified with a bioorthogonal functional group (chemical reporter) and introduced to the cell; substrates include metabolites, enzyme inhibitors, etc. The chemical reporter must not alter the structure of the substrate dramatically to avoid affecting its bioactivity. Secondly, a probe containing the complementary functional group is introduced to react and label the substrate.Although effective bioorthogonal reactions such as copper-free click chemistry have been developed, development of new reactions continues to generate orthogonal methods for labeling to allow multiple methods of labeling to be used in the same biosystems.