Chemistry 121: Topic 2 - From Atoms to Stoichiometry Topic 2
... ¾ Compounds are composed of atoms of more than one element. In any compound, the ratio of the numbers of atoms of any two of the elements present is either an integer or a simple fraction. ¾ A chemical reaction involves only the separation, combination, or rearrangement of atoms; it does not result ...
... ¾ Compounds are composed of atoms of more than one element. In any compound, the ratio of the numbers of atoms of any two of the elements present is either an integer or a simple fraction. ¾ A chemical reaction involves only the separation, combination, or rearrangement of atoms; it does not result ...
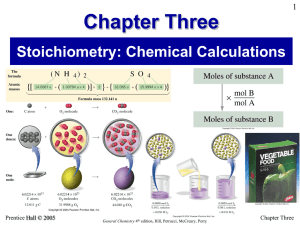
Chapter Three
... sodium, 22.67% sulfur, and 45.02% oxygen. Find the empirical formula for this compound. • HINT = Whenever you are given %s, we assume we ALWAYS have a 100-gram sample, so immediately convert you %s to grams. ...
... sodium, 22.67% sulfur, and 45.02% oxygen. Find the empirical formula for this compound. • HINT = Whenever you are given %s, we assume we ALWAYS have a 100-gram sample, so immediately convert you %s to grams. ...
CHAPTER
... In Chapter 11, you learned that volumes of gases always combine in definite ratios. This observation, called the law of combining volumes, is based on measurements of the gas volumes. When Avogadro suggested that gases combine in fixed ratios because equal volumes of gases at the same temperature an ...
... In Chapter 11, you learned that volumes of gases always combine in definite ratios. This observation, called the law of combining volumes, is based on measurements of the gas volumes. When Avogadro suggested that gases combine in fixed ratios because equal volumes of gases at the same temperature an ...
Physical Science Semester 2 Final Exam Review Questions
... A.Radioactive isotopes release a large amount of chemical energy. B.When large atoms break apart, some of their mass is converted to energy. C. The bonds between uranium atoms are very strong and release energy when they are broken. D.A significant amount of energy is released when two atoms come to ...
... A.Radioactive isotopes release a large amount of chemical energy. B.When large atoms break apart, some of their mass is converted to energy. C. The bonds between uranium atoms are very strong and release energy when they are broken. D.A significant amount of energy is released when two atoms come to ...
effect of inorganic ions on the oxidation of dichlorvos insecticide with
... first stage is 15.8%, as seen in Figure 2. It may be derived that FeH,PO,+ possibly reacts with hydrogen peroxide and produces radicals. It is still possible to oxidize dichlorvos in the first stage. In the second stage, FeH,P0,2’reacts ...
... first stage is 15.8%, as seen in Figure 2. It may be derived that FeH,PO,+ possibly reacts with hydrogen peroxide and produces radicals. It is still possible to oxidize dichlorvos in the first stage. In the second stage, FeH,P0,2’reacts ...
Complete Set
... increasing levels of CO2 will lead to increased dissolution of CaCO3 and critically affect the survival of life forms that rely on a carbonaceous skeleton. Calculate the concentrations of Ca2+ and CO32– in a saturated solution of CaCO3. (The Ksp of CaCO3 is 3.3 × 10–9.) The dissolution of CaCO3 foll ...
... increasing levels of CO2 will lead to increased dissolution of CaCO3 and critically affect the survival of life forms that rely on a carbonaceous skeleton. Calculate the concentrations of Ca2+ and CO32– in a saturated solution of CaCO3. (The Ksp of CaCO3 is 3.3 × 10–9.) The dissolution of CaCO3 foll ...
CHEMISTRY CET
... Volume of 0.03 M KMnO4 required to oxidize the same amount of H2S gas to sulphur, in acidic medium is 1) 80 cm3 2) 120 cm3 3) 60 cm3 4) 90 cm3 Solution : K2Cl2O4 KMnO4 ...
... Volume of 0.03 M KMnO4 required to oxidize the same amount of H2S gas to sulphur, in acidic medium is 1) 80 cm3 2) 120 cm3 3) 60 cm3 4) 90 cm3 Solution : K2Cl2O4 KMnO4 ...
Synthesis and Characterisation of N
... I hereby certify that I am the sole author of this thesis and that no part of this thesis has been published or submitted for publication. I certify that, to the best of my knowledge, my thesis does not infringe upon anyone’s copyright nor violate any proprietary rights and that any ideas, technique ...
... I hereby certify that I am the sole author of this thesis and that no part of this thesis has been published or submitted for publication. I certify that, to the best of my knowledge, my thesis does not infringe upon anyone’s copyright nor violate any proprietary rights and that any ideas, technique ...
Tro Chemistry a Molecular Approach, 3E
... (P, V, and T) defines the state (or macrostate) of the system. As long as these conditions remain constant, the energy of the system also remains constant. However, exactly where that energy is at any given instant is anything but constant. At any one instant, a particular gas particle may have lots ...
... (P, V, and T) defines the state (or macrostate) of the system. As long as these conditions remain constant, the energy of the system also remains constant. However, exactly where that energy is at any given instant is anything but constant. At any one instant, a particular gas particle may have lots ...
Chemical Equilibrium
... Before we use the relevant equation, we need to do two things: convert the temperature to kelvins and determine Δn. Converting the temperature is easy: ...
... Before we use the relevant equation, we need to do two things: convert the temperature to kelvins and determine Δn. Converting the temperature is easy: ...
Class XI Physical Chemistry Short note
... All atoms of a given element are identical. The atoms of a given element are different from those of any ...
... All atoms of a given element are identical. The atoms of a given element are different from those of any ...
Laboratory Manual
... the lab. I recognize my responsibility to follow these practices and precautions while I am present in the laboratory. When I am in the laboratory I will wear the recommended eye and personal protection; I will follow the recommended procedures for working safely in the laboratory. ...
... the lab. I recognize my responsibility to follow these practices and precautions while I am present in the laboratory. When I am in the laboratory I will wear the recommended eye and personal protection; I will follow the recommended procedures for working safely in the laboratory. ...
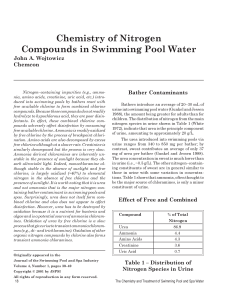
w_4-3 Chemistry of Nitrogen Compounds
... to chloroform), it will tend to volatilize into the atmosphere, thereby reducing the concentration in the water. This will be most pronounced in spas with their higher temperatures and use of aeration. Nitrogen trichloride is also decomposed by sunlight and it can undergo slow decomposition by hydro ...
... to chloroform), it will tend to volatilize into the atmosphere, thereby reducing the concentration in the water. This will be most pronounced in spas with their higher temperatures and use of aeration. Nitrogen trichloride is also decomposed by sunlight and it can undergo slow decomposition by hydro ...
Chapter 3 - Educator
... Once we know the formulas of the reactants and products in a reaction, we can write the unbalanced equation. We then balance the equation by determining the coefficients that provide equal numbers of each type of atom on each side of the equation. For most purposes, a balanced equation should contai ...
... Once we know the formulas of the reactants and products in a reaction, we can write the unbalanced equation. We then balance the equation by determining the coefficients that provide equal numbers of each type of atom on each side of the equation. For most purposes, a balanced equation should contai ...
Chapter 5 Thermochemistry
... A bowler lifts a 5.4-kg (12-lb) bowling ball from ground level to a height of 1.6 m (5.2 ft) and then drops it. (a) What happens to the potential energy of the ball as it is raised? (b) What quantity of work, in J, is used to raise the ball? (c) After the ball is dropped, it gains kinetic energy. If ...
... A bowler lifts a 5.4-kg (12-lb) bowling ball from ground level to a height of 1.6 m (5.2 ft) and then drops it. (a) What happens to the potential energy of the ball as it is raised? (b) What quantity of work, in J, is used to raise the ball? (c) After the ball is dropped, it gains kinetic energy. If ...
paper - General Atomics Fusion Group
... question about the technical feasibility and the potential for high efficiency, cycles with proven low cost and high efficiency have yet to be developed commercially. Over one hundred cycles have been proposed, but substantial research has been executed on only a few. This report describes work acco ...
... question about the technical feasibility and the potential for high efficiency, cycles with proven low cost and high efficiency have yet to be developed commercially. Over one hundred cycles have been proposed, but substantial research has been executed on only a few. This report describes work acco ...
Specification – AS/A Level Chemistry A
... (ii) Chemistry (a) Structure and Bonding The outer electrons of atoms are involved in chemical reactions. The structure and properties of a substance are strongly dependent on the nature of the bonding that results from the forces between the electrons and nuclei of atoms. ...
... (ii) Chemistry (a) Structure and Bonding The outer electrons of atoms are involved in chemical reactions. The structure and properties of a substance are strongly dependent on the nature of the bonding that results from the forces between the electrons and nuclei of atoms. ...
expected output
... -Explain the gas laws and relate them to the Kinetic molecular theory of gases. -Calculate the different types of equilibrium constants using balanced equations and given species concentrations. -Explain what is pH and how it can be measured and calculated. -Explain how to make a buffer solution of ...
... -Explain the gas laws and relate them to the Kinetic molecular theory of gases. -Calculate the different types of equilibrium constants using balanced equations and given species concentrations. -Explain what is pH and how it can be measured and calculated. -Explain how to make a buffer solution of ...
Quantum Tunnelling to the Origin and Evolution of Life
... under certain conditions elementary particles, nucleons or atoms are able to negotiate the obstacle of a potential barrier (which is, from the classical point of view, a forbidden area for a particle) without having the energy to overcome it. The STM setup gives an illustrative example of potential ...
... under certain conditions elementary particles, nucleons or atoms are able to negotiate the obstacle of a potential barrier (which is, from the classical point of view, a forbidden area for a particle) without having the energy to overcome it. The STM setup gives an illustrative example of potential ...
Bioorthogonal chemistry
The term bioorthogonal chemistry refers to any chemical reaction that can occur inside of living systems without interfering with native biochemical processes. The term was coined by Carolyn R. Bertozzi in 2003. Since its introduction, the concept of the bioorthogonal reaction has enabled the study of biomolecules such as glycans, proteins, and lipids in real time in living systems without cellular toxicity. A number of chemical ligation strategies have been developed that fulfill the requirements of bioorthogonality, including the 1,3-dipolar cycloaddition between azides and cyclooctynes (also termed copper-free click chemistry), between nitrones and cyclooctynes, oxime/hydrazone formation from aldehydes and ketones, the tetrazine ligation, the isocyanide-based click reaction, and most recently, the quadricyclane ligation.The use of bioorthogonal chemistry typically proceeds in two steps. First, a cellular substrate is modified with a bioorthogonal functional group (chemical reporter) and introduced to the cell; substrates include metabolites, enzyme inhibitors, etc. The chemical reporter must not alter the structure of the substrate dramatically to avoid affecting its bioactivity. Secondly, a probe containing the complementary functional group is introduced to react and label the substrate.Although effective bioorthogonal reactions such as copper-free click chemistry have been developed, development of new reactions continues to generate orthogonal methods for labeling to allow multiple methods of labeling to be used in the same biosystems.