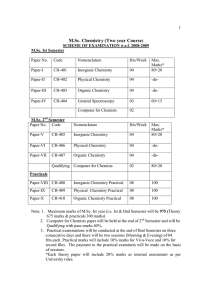
Appendix
... 1. Which of the following condiments do you think has the lowest salt content? a. mustard ...
... 1. Which of the following condiments do you think has the lowest salt content? a. mustard ...
Problem Set 7
... 48) Define Excess Reactant and Limiting Reactant. Why are these two terms important in industrial production of compounds? The reactant is excess is the substance that remains after the limiting reactant runs out. The limiting reactant is the reactant that runs out before any other in a chemical rea ...
... 48) Define Excess Reactant and Limiting Reactant. Why are these two terms important in industrial production of compounds? The reactant is excess is the substance that remains after the limiting reactant runs out. The limiting reactant is the reactant that runs out before any other in a chemical rea ...
CHAPTER 21 ELECTROCHEMISTRY: CHEMICAL CHANGE AND
... Add half-reactions and cancel substances that appear as both reactants and products 2 MnO4−(aq) + 8 H+(aq) + 3 SO32−(aq) + 3 H2O(l) → 2 MnO2(s) + 4 H2O(l) + 3 SO42−(aq) + 6 H+(aq) The balanced equation in acidic solution is: 2 MnO4−(aq) + 2 H+(aq) + 3 SO32−(aq) → 2 MnO2(s) + H2O(l) + 3 SO42−(aq) To ...
... Add half-reactions and cancel substances that appear as both reactants and products 2 MnO4−(aq) + 8 H+(aq) + 3 SO32−(aq) + 3 H2O(l) → 2 MnO2(s) + 4 H2O(l) + 3 SO42−(aq) + 6 H+(aq) The balanced equation in acidic solution is: 2 MnO4−(aq) + 2 H+(aq) + 3 SO32−(aq) → 2 MnO2(s) + H2O(l) + 3 SO42−(aq) To ...
Chapter 22 - 2012 Book Archive
... so its chemistry is more diverse than that of groups 1 and 2, which include only metallic elements. Except for the lightest element (boron), the group 13 elements are all relatively electropositive; that is, they tend to lose electrons in chemical reactions rather than gain them. Although group 13 i ...
... so its chemistry is more diverse than that of groups 1 and 2, which include only metallic elements. Except for the lightest element (boron), the group 13 elements are all relatively electropositive; that is, they tend to lose electrons in chemical reactions rather than gain them. Although group 13 i ...
L A B O
... 3. Do not eat or drink in the laboratory. 4. Do not taste any chemical. 5. Do not smell any chemicals directly. Use your fingers to waft the odor to your nose. 6. Do not pipet solutions by mouth. Rubber pipet bulbs are provided at each lab station. 7. Do not put flammable liquids near an open flame. ...
... 3. Do not eat or drink in the laboratory. 4. Do not taste any chemical. 5. Do not smell any chemicals directly. Use your fingers to waft the odor to your nose. 6. Do not pipet solutions by mouth. Rubber pipet bulbs are provided at each lab station. 7. Do not put flammable liquids near an open flame. ...
Stoichiometry: Calculations with Chemical Formulas and
... # moles reactant A / coefficient A = x # moles reactant B / coefficent B = y Step 3: The smaller # (x or y) identifies the Limiting Reagent. Step 4: Use the # of moles of the Limiting Reagent to solve the rest of the problem. Stoichiometry © 2009, Prentice-Hall, Inc. ...
... # moles reactant A / coefficient A = x # moles reactant B / coefficent B = y Step 3: The smaller # (x or y) identifies the Limiting Reagent. Step 4: Use the # of moles of the Limiting Reagent to solve the rest of the problem. Stoichiometry © 2009, Prentice-Hall, Inc. ...
2 - OnCourse
... In the molecule CO2, there is one atom of carbon. The subscript on the symbol for oxygen tells us that there are 2 atoms of oxygen in the molecule. There is one mole of carbon in CO2 There are two moles of oxygen in CO2 The molar mass of CO2 is 1 x 12.01 g C / mol CO2 ...
... In the molecule CO2, there is one atom of carbon. The subscript on the symbol for oxygen tells us that there are 2 atoms of oxygen in the molecule. There is one mole of carbon in CO2 There are two moles of oxygen in CO2 The molar mass of CO2 is 1 x 12.01 g C / mol CO2 ...
CLUE - virtual laboratories
... many times do we hear about “natural remedies, without drugs or chemicals,” despite the fact that everything is composed of chemicals and the most toxic chemicals known are natural products.2 A growing body of research results on student understanding of chemistry indicates, pretty emphatically, tha ...
... many times do we hear about “natural remedies, without drugs or chemicals,” despite the fact that everything is composed of chemicals and the most toxic chemicals known are natural products.2 A growing body of research results on student understanding of chemistry indicates, pretty emphatically, tha ...
SCH3U: Final Exam Review
... calculate the mass of this precipitate that is formed. 44. An excess of sodium carbonate solution is added to 75.0 mL of calcium chloride solution. 7.50 g of precipitate is formed. Calculate the concentration of the calcium chloride solution. 45. Suppose that you are given a sample that contains Ag+ ...
... calculate the mass of this precipitate that is formed. 44. An excess of sodium carbonate solution is added to 75.0 mL of calcium chloride solution. 7.50 g of precipitate is formed. Calculate the concentration of the calcium chloride solution. 45. Suppose that you are given a sample that contains Ag+ ...
1970 - 2005 Solids/Liquids/Solutions FRQs
... Lattice energy - quantity of energy released in the formation of one mole of an ionic solid from its separated gaseous ions. The energy quantities needed to be determined: sublimation of solid metal ionization of gaseous atomic metal (ionization energy) dissociation of gaseous non-metal ion formatio ...
... Lattice energy - quantity of energy released in the formation of one mole of an ionic solid from its separated gaseous ions. The energy quantities needed to be determined: sublimation of solid metal ionization of gaseous atomic metal (ionization energy) dissociation of gaseous non-metal ion formatio ...
1970 - Warren County Schools
... Lattice energy - quantity of energy released in the formation of one mole of an ionic solid from its separated gaseous ions. The energy quantities needed to be determined: sublimation of solid metal ionization of gaseous atomic metal (ionization energy) dissociation of gaseous non-metal ion formatio ...
... Lattice energy - quantity of energy released in the formation of one mole of an ionic solid from its separated gaseous ions. The energy quantities needed to be determined: sublimation of solid metal ionization of gaseous atomic metal (ionization energy) dissociation of gaseous non-metal ion formatio ...
LABORATORY MANUAL FOR GENERAL CHEMISTRY I
... 3. Do not eat or drink in the laboratory. 4. Do not taste any chemical. 5. Do not smell any chemicals directly. Use your fingers to waft the odor to your nose. 6. Do not pipet solutions by mouth. Rubber pipet bulbs are provided at each lab station. 7. Do not put flammable liquids near an open flame. ...
... 3. Do not eat or drink in the laboratory. 4. Do not taste any chemical. 5. Do not smell any chemicals directly. Use your fingers to waft the odor to your nose. 6. Do not pipet solutions by mouth. Rubber pipet bulbs are provided at each lab station. 7. Do not put flammable liquids near an open flame. ...
The shock tube as wave reactor for kinetic studies and material
... reaction complexity involved and the dif®culties in diagnostics. A pre-condition for this type of experimentation is that the solid phase (particles) are homogeneously dispersed in the carrier gas. Interference between the complexities of two-phase ¯ow and kinetics is another problem to be overcome ...
... reaction complexity involved and the dif®culties in diagnostics. A pre-condition for this type of experimentation is that the solid phase (particles) are homogeneously dispersed in the carrier gas. Interference between the complexities of two-phase ¯ow and kinetics is another problem to be overcome ...
chemistry - The Aga Khan University
... 13.3.1 Atomic and Physical Properties 13.3.1.1 Trends in Atomic Radius 13.3.1.2 Trends in First Ionization Energy 13.3.1.3 Trends in Electronegativity 13.3.1.4 Trends in Melting and Boiling Points 13.3.2 Trends in Reactivity with Water 13.3.3 Reactions with Oxygen and Nitrogen 13.3.3.1 Simple Oxides ...
... 13.3.1 Atomic and Physical Properties 13.3.1.1 Trends in Atomic Radius 13.3.1.2 Trends in First Ionization Energy 13.3.1.3 Trends in Electronegativity 13.3.1.4 Trends in Melting and Boiling Points 13.3.2 Trends in Reactivity with Water 13.3.3 Reactions with Oxygen and Nitrogen 13.3.3.1 Simple Oxides ...
chap15pptlecture_chapte.ppt [Read-Only]
... If a reaction can be expressed as the sum of two or more reactions, the equilibrium constant for the overall reaction is given by the product of the equilibrium constants of the individual reactions. ...
... If a reaction can be expressed as the sum of two or more reactions, the equilibrium constant for the overall reaction is given by the product of the equilibrium constants of the individual reactions. ...
Stoichiometry: Calculations with Chemical Formulas and
... Sample Exercise 3.1 Interpreting and Balancing Chemical Equations The following diagram represents a chemical reaction in which the red spheres are oxygen atoms and the blue spheres are nitrogen atoms. (a) Write the chemical formulas for the reactants and products. (b) Write a balanced equation for ...
... Sample Exercise 3.1 Interpreting and Balancing Chemical Equations The following diagram represents a chemical reaction in which the red spheres are oxygen atoms and the blue spheres are nitrogen atoms. (a) Write the chemical formulas for the reactants and products. (b) Write a balanced equation for ...
Rh(acac)(CO)(PR1R2R3) - University of the Free State
... Rhodium is often used as an alloying agent to harden platinum and palladium. It is used in electrical contact material, due to its low electrical resistance, and in optical instruments and jewellery because of its high reflectance and hardness. It is extensively used in chemical synthesis as an impo ...
... Rhodium is often used as an alloying agent to harden platinum and palladium. It is used in electrical contact material, due to its low electrical resistance, and in optical instruments and jewellery because of its high reflectance and hardness. It is extensively used in chemical synthesis as an impo ...
the chemical and physical properties of condensed
... more useful than the polyphosphate glasses and should be explored in greater detail during the next decade. Several areas deserve more attention than they have received. The three component M2O—MO—P2O5 phase systems should contain stable polyphosphates in the penta- to decaphosphate range. The curre ...
... more useful than the polyphosphate glasses and should be explored in greater detail during the next decade. Several areas deserve more attention than they have received. The three component M2O—MO—P2O5 phase systems should contain stable polyphosphates in the penta- to decaphosphate range. The curre ...
Post Lab Questions
... In general most missed work is due on the next Friday. For any planned absence work that is due during the absence must be turned in before your departure. Laboratories, quizzes, and exams cannot be made up unless prior arrangements in writing have been made. If you make prior arrangements and miss ...
... In general most missed work is due on the next Friday. For any planned absence work that is due during the absence must be turned in before your departure. Laboratories, quizzes, and exams cannot be made up unless prior arrangements in writing have been made. If you make prior arrangements and miss ...
Bioorthogonal chemistry
The term bioorthogonal chemistry refers to any chemical reaction that can occur inside of living systems without interfering with native biochemical processes. The term was coined by Carolyn R. Bertozzi in 2003. Since its introduction, the concept of the bioorthogonal reaction has enabled the study of biomolecules such as glycans, proteins, and lipids in real time in living systems without cellular toxicity. A number of chemical ligation strategies have been developed that fulfill the requirements of bioorthogonality, including the 1,3-dipolar cycloaddition between azides and cyclooctynes (also termed copper-free click chemistry), between nitrones and cyclooctynes, oxime/hydrazone formation from aldehydes and ketones, the tetrazine ligation, the isocyanide-based click reaction, and most recently, the quadricyclane ligation.The use of bioorthogonal chemistry typically proceeds in two steps. First, a cellular substrate is modified with a bioorthogonal functional group (chemical reporter) and introduced to the cell; substrates include metabolites, enzyme inhibitors, etc. The chemical reporter must not alter the structure of the substrate dramatically to avoid affecting its bioactivity. Secondly, a probe containing the complementary functional group is introduced to react and label the substrate.Although effective bioorthogonal reactions such as copper-free click chemistry have been developed, development of new reactions continues to generate orthogonal methods for labeling to allow multiple methods of labeling to be used in the same biosystems.

















![chap15pptlecture_chapte.ppt [Read-Only]](http://s1.studyres.com/store/data/015369082_1-00cbf06a2d468a4ae1c963f5ca674e31-300x300.png)