BSc Honours chemistry CBCS Syllabus 2016-17
... expression for lattice energy.Madelung constant, Born-Haber cycle and its application, Solvation energy. (ii) Covalent bond: Lewis structure, Valence Bond theory (Heitler-London approach). Energetics of hybridization, equivalent and non-equivalent hybrid orbitals.Bent’s rule, Resonance and resonance ...
... expression for lattice energy.Madelung constant, Born-Haber cycle and its application, Solvation energy. (ii) Covalent bond: Lewis structure, Valence Bond theory (Heitler-London approach). Energetics of hybridization, equivalent and non-equivalent hybrid orbitals.Bent’s rule, Resonance and resonance ...
Solubility Equilibria
... Calculate the equilibrium concentrations of Pb2+ and I‒ ions in a solution formed by mixing 100 0 mL of 0.0500 M of 0 0500 M in a solution formed by mixing 100.0 mL Pb(NO3)2 and 200.0 mL of 0.100 M NaI solutions. The Ksp for PbI2 is 1.4 10‐8 . ...
... Calculate the equilibrium concentrations of Pb2+ and I‒ ions in a solution formed by mixing 100 0 mL of 0.0500 M of 0 0500 M in a solution formed by mixing 100.0 mL Pb(NO3)2 and 200.0 mL of 0.100 M NaI solutions. The Ksp for PbI2 is 1.4 10‐8 . ...
Chapter 15: Chemical Equilibrium
... When this equilibrium state is achieved, the concentrations of all the species in solution are constant, even though the forward and reverse reactions continue to take place. Note that when a system is at equilibrium, while the rates of the forward and reverse reactions are equal, the rate constants ...
... When this equilibrium state is achieved, the concentrations of all the species in solution are constant, even though the forward and reverse reactions continue to take place. Note that when a system is at equilibrium, while the rates of the forward and reverse reactions are equal, the rate constants ...
Regents Chemistry Review - New York Science Teacher
... In the laboratory, a glass tube is filled with hydrogen gas at a very low pressure. When a scientist applies a high voltage between metal electrodes in the tube, light .is emitted. When the light is analyzed with a spectroscope four distinct spectral lines are noted. Information on their frequency ...
... In the laboratory, a glass tube is filled with hydrogen gas at a very low pressure. When a scientist applies a high voltage between metal electrodes in the tube, light .is emitted. When the light is analyzed with a spectroscope four distinct spectral lines are noted. Information on their frequency ...
Multiple Choice Exam Review June 2016
... ____ 34. Catalysts can be used to speed up a reaction. _________________________ ____ 35. The value of the rate constant, k, is valid only for a specific reaction at a specific temperature. _________________________ ____ 36. An ineffective collision is one that has sufficient energy and correct orie ...
... ____ 34. Catalysts can be used to speed up a reaction. _________________________ ____ 35. The value of the rate constant, k, is valid only for a specific reaction at a specific temperature. _________________________ ____ 36. An ineffective collision is one that has sufficient energy and correct orie ...
Soln Chem 2008Nov(9746)
... Boiling point of Group VII elements increases from Cl2 to I2 due to stronger intermolecular van der Waals' forces as the number of electrons increases from Cl2 to I2. From Cl to I, electron affinity becomes less negative due to the increase in atomic size and hence, weaker attraction for the additio ...
... Boiling point of Group VII elements increases from Cl2 to I2 due to stronger intermolecular van der Waals' forces as the number of electrons increases from Cl2 to I2. From Cl to I, electron affinity becomes less negative due to the increase in atomic size and hence, weaker attraction for the additio ...
The Role of Medicinal Chemistry in Canadian Pharmacy
... 2735 B.C. – Babylonian, Chinese, Indian cultures 400 B.C. – Greek culture (ancient apothecary) ...
... 2735 B.C. – Babylonian, Chinese, Indian cultures 400 B.C. – Greek culture (ancient apothecary) ...
chapter 20 - United International College
... through which the cations and anions can move from one electrode compartment to the other. This requirement is satisfied by a salt bridge, which, in its simplest form, is an inverted U tube containing an inert electrolyte solution, such as KCl or NH4NO3, whose ions will not react with other ions in ...
... through which the cations and anions can move from one electrode compartment to the other. This requirement is satisfied by a salt bridge, which, in its simplest form, is an inverted U tube containing an inert electrolyte solution, such as KCl or NH4NO3, whose ions will not react with other ions in ...
kinetic characterisation of catalysts for methanol synthesis
... activity and durability an interest to develop better catalyst has steadily been observed. In order to improve the efficiency of the process, modified catalysts are examined. The Cu/ZnO/ZrO2 catalyst and the addition of B, Ga, In, Gd, Y, Mn and Mg oxides were studied by Skrzypek et al. (2006). The a ...
... activity and durability an interest to develop better catalyst has steadily been observed. In order to improve the efficiency of the process, modified catalysts are examined. The Cu/ZnO/ZrO2 catalyst and the addition of B, Ga, In, Gd, Y, Mn and Mg oxides were studied by Skrzypek et al. (2006). The a ...
Ans:- (i) Gluconic acid - Kendriya Vidyalaya No.2, Kribhco, Surat
... Q-7 How does an electrochemical cell help in predicting the feasibility of a redox reaction ? Ans-14.If E0 of the cell is +ve it will yield –ve ∆G0 Value which indicates the reaction is spontaneous. ∆G0 = -n F E0 Q.8 Why m for acetic acid cannot be determined experimentally? Ans. Molar conductiv ...
... Q-7 How does an electrochemical cell help in predicting the feasibility of a redox reaction ? Ans-14.If E0 of the cell is +ve it will yield –ve ∆G0 Value which indicates the reaction is spontaneous. ∆G0 = -n F E0 Q.8 Why m for acetic acid cannot be determined experimentally? Ans. Molar conductiv ...
PDF - mockies – Mockiesgateacademy
... Where has chemistry come from ? Throughout the history of the human race, people have struggled to make sense of the world around them. Through the branch of science we call chemistry we have gained an understanding of the matter which makes up our world and of the interactions between particles on ...
... Where has chemistry come from ? Throughout the history of the human race, people have struggled to make sense of the world around them. Through the branch of science we call chemistry we have gained an understanding of the matter which makes up our world and of the interactions between particles on ...
UNIVERSITY OF DELHI FACULTY OF SCIENCE SYLLABUS OF COURSES TO BE OFFERED
... The CBCS provides an opportunity for the students to choose courses from the prescribed courses comprising core, elective/minor or skill based courses. The courses can be evaluated following the grading system, which is considered to be better than the conventional marks system. Therefore, it is nec ...
... The CBCS provides an opportunity for the students to choose courses from the prescribed courses comprising core, elective/minor or skill based courses. The courses can be evaluated following the grading system, which is considered to be better than the conventional marks system. Therefore, it is nec ...
Chapter 3 2014
... 2 atoms of Al and 3 molecules of (SO4)2- = 1 formula unit Al2(SO4)3 2 moles of Al and 3 moles of (SO4)2- = 1 formula unit Al2(SO4)3 1 formula unit Al2(SO4)3 = 342.17 amu Al2(SO4)3 1 mole Al2(SO4)3 = 342.17 g Al2(SO4)3 1 mole Al2(SO4)3 = 6.022 x 1023 formula units Al2(SO4)3 1 mole Al2(SO4)3 = 2 mol A ...
... 2 atoms of Al and 3 molecules of (SO4)2- = 1 formula unit Al2(SO4)3 2 moles of Al and 3 moles of (SO4)2- = 1 formula unit Al2(SO4)3 1 formula unit Al2(SO4)3 = 342.17 amu Al2(SO4)3 1 mole Al2(SO4)3 = 342.17 g Al2(SO4)3 1 mole Al2(SO4)3 = 6.022 x 1023 formula units Al2(SO4)3 1 mole Al2(SO4)3 = 2 mol A ...
KCET – CHEMISTRY – 2016 - Medicine.careers360.com
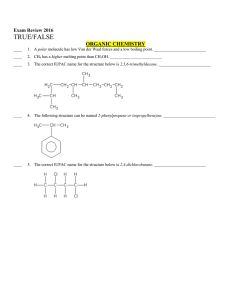
... 16. Benezene carbaldehyde is reacted with concentrated NaOH solution to give the products A and B. The product A can be used food preservative and the product B is an aromatic hydroxyl compound where OH group is linked to sp3 hydridised carbon atom next to Benzene ring. The products A and B respect ...
... 16. Benezene carbaldehyde is reacted with concentrated NaOH solution to give the products A and B. The product A can be used food preservative and the product B is an aromatic hydroxyl compound where OH group is linked to sp3 hydridised carbon atom next to Benzene ring. The products A and B respect ...
LABORATORY MANUAL FOR CHEMISTRY 102
... to the sum of x + y + z +... .) The term k is known as the rate constant for the reaction. Usually, when a reaction is initiated, the rate (known as the initial rate) is found to be at its maximum value. As the reaction progresses, reactants are consumed (lowering their concentrations) and the rate ...
... to the sum of x + y + z +... .) The term k is known as the rate constant for the reaction. Usually, when a reaction is initiated, the rate (known as the initial rate) is found to be at its maximum value. As the reaction progresses, reactants are consumed (lowering their concentrations) and the rate ...
Kinetic isotope effects of 12CH3D+OH and 13CH3D+OH from 278 to
... spectroscopy (Ono et al., 2014; Wang et al., 2015) facilitate measurement of rare doubly substituted isotopologues. The abundance of these “clumped” isotopologues (clumped refers to the rare isotopes being clumped together) generally follows a stochastic distribution (i.e., [12 CH4 ][13 CH3 D] = [13 ...
... spectroscopy (Ono et al., 2014; Wang et al., 2015) facilitate measurement of rare doubly substituted isotopologues. The abundance of these “clumped” isotopologues (clumped refers to the rare isotopes being clumped together) generally follows a stochastic distribution (i.e., [12 CH4 ][13 CH3 D] = [13 ...
Vinnitsa National Pirogov Memorial Medical University Biological
... 1.Write the electronic structure of potassium atom and ion. 2. Write the electronic structure of aluminium atom and Al3+ ion. 3.Write the equations of the below given chain. ...
... 1.Write the electronic structure of potassium atom and ion. 2. Write the electronic structure of aluminium atom and Al3+ ion. 3.Write the equations of the below given chain. ...
teaching and learning materials - UNESDOC
... organisations and donors, has attempted to address and overcome that situation. The programme has promoted the idea that practical experiences can be provided at much lower cost and with much lower environmental impact by working on a small scale and using less expensive materials. The programme has ...
... organisations and donors, has attempted to address and overcome that situation. The programme has promoted the idea that practical experiences can be provided at much lower cost and with much lower environmental impact by working on a small scale and using less expensive materials. The programme has ...
Unit 7: Reduction, Oxidation and Electrochemistry
... Unit 7: Reduction, Oxidation and Electrochemistry Chapter 17: Electrochemistry 4.9: Oxidation-Reduction Reactions Reduction-Oxidation Reactions (Redox Rxn): - chemical reactions where there is a transfer of electron(s). Oxidation States (Oxidation Number): - a number that is arbitrary assigned to an ...
... Unit 7: Reduction, Oxidation and Electrochemistry Chapter 17: Electrochemistry 4.9: Oxidation-Reduction Reactions Reduction-Oxidation Reactions (Redox Rxn): - chemical reactions where there is a transfer of electron(s). Oxidation States (Oxidation Number): - a number that is arbitrary assigned to an ...
THESE DOCTORAT DE L`UNIVERSITE DE TOULOUSE ET
... compounds [Cp*2M2O5] (M=Mo, W) are described in the thesis. Subsequently, the reactivity of the WVI complex with sulphur donor ligands is presented. This comprises the investigation of the interaction between [Cp*2W2O5] and mercaptocarboxylic acids, especially 3-mercaptopropionic acid, which resulte ...
... compounds [Cp*2M2O5] (M=Mo, W) are described in the thesis. Subsequently, the reactivity of the WVI complex with sulphur donor ligands is presented. This comprises the investigation of the interaction between [Cp*2W2O5] and mercaptocarboxylic acids, especially 3-mercaptopropionic acid, which resulte ...
Bioorthogonal chemistry
The term bioorthogonal chemistry refers to any chemical reaction that can occur inside of living systems without interfering with native biochemical processes. The term was coined by Carolyn R. Bertozzi in 2003. Since its introduction, the concept of the bioorthogonal reaction has enabled the study of biomolecules such as glycans, proteins, and lipids in real time in living systems without cellular toxicity. A number of chemical ligation strategies have been developed that fulfill the requirements of bioorthogonality, including the 1,3-dipolar cycloaddition between azides and cyclooctynes (also termed copper-free click chemistry), between nitrones and cyclooctynes, oxime/hydrazone formation from aldehydes and ketones, the tetrazine ligation, the isocyanide-based click reaction, and most recently, the quadricyclane ligation.The use of bioorthogonal chemistry typically proceeds in two steps. First, a cellular substrate is modified with a bioorthogonal functional group (chemical reporter) and introduced to the cell; substrates include metabolites, enzyme inhibitors, etc. The chemical reporter must not alter the structure of the substrate dramatically to avoid affecting its bioactivity. Secondly, a probe containing the complementary functional group is introduced to react and label the substrate.Although effective bioorthogonal reactions such as copper-free click chemistry have been developed, development of new reactions continues to generate orthogonal methods for labeling to allow multiple methods of labeling to be used in the same biosystems.