chapter 2 - chemical equations and reaction yields
... The following chemical equations can be balanced by “inspection.” The first two are the answers alone. For (c) and (d) the method is developed as in Example 2–1. (a) N2 + O2 → 2 NO (b) 2 N2 + O2 → 2 N2O (c) ___ K2SO3 + ___ HCl → ___ KCl + ___ H2O + ___SO2 The key to this method is to avoid “traps” t ...
... The following chemical equations can be balanced by “inspection.” The first two are the answers alone. For (c) and (d) the method is developed as in Example 2–1. (a) N2 + O2 → 2 NO (b) 2 N2 + O2 → 2 N2O (c) ___ K2SO3 + ___ HCl → ___ KCl + ___ H2O + ___SO2 The key to this method is to avoid “traps” t ...
Novel Methods and Materials in Development of Liquid Carrier
... Melin. It was clear that I would become the member of the „fabulous” membrane group working on gas and vapour separations with membranes. In the beginning, I should have worked on the improvement of inorganic membranes, in particular in pin-hole closure of zeolite and silica membranes using procedur ...
... Melin. It was clear that I would become the member of the „fabulous” membrane group working on gas and vapour separations with membranes. In the beginning, I should have worked on the improvement of inorganic membranes, in particular in pin-hole closure of zeolite and silica membranes using procedur ...
Chemistry 133 Problem Set Introduction
... 1.23 Define a pure substance and a mixture. Give an example of each. 1.24 Give two examples of each of the following. (a) an element (b) a compound (c) a homogeneous mixture (d) a heterogeneous mixture 1.25 What experiments could you perform to demonstrate that liquid water and ice contain the same ...
... 1.23 Define a pure substance and a mixture. Give an example of each. 1.24 Give two examples of each of the following. (a) an element (b) a compound (c) a homogeneous mixture (d) a heterogeneous mixture 1.25 What experiments could you perform to demonstrate that liquid water and ice contain the same ...
Stoichiometry - Mr Field's Chemistry Class
... A good tip for students to improve understanding of the calculations is to get them to highlight numbers in the question and through the maths in different colours so they can see where numbers are coming from and going to. ...
... A good tip for students to improve understanding of the calculations is to get them to highlight numbers in the question and through the maths in different colours so they can see where numbers are coming from and going to. ...
Surface chemistry of carbon dioxide - Max-Planck
... the 2n u orbital. The six occupied orbitals may be classified as two cy-C-O bonds (3cyg,2%) and two lone pairs (4%, 3%). The lnu represents the n C - O bonds and lng the n lone pairs. In order to judge the stability of the linear geometry, Fig. 1 shows, in a qualitative way, the energy positions of ...
... the 2n u orbital. The six occupied orbitals may be classified as two cy-C-O bonds (3cyg,2%) and two lone pairs (4%, 3%). The lnu represents the n C - O bonds and lng the n lone pairs. In order to judge the stability of the linear geometry, Fig. 1 shows, in a qualitative way, the energy positions of ...
Slide 1
... Reversible reactions • You can recognize that the ammonia-forming reaction reaches a state of chemical equilibrium because its chemical equation is written with a double arrow like this. • At equilibrium, the concentrations of reactants and products are constant. • However, that does not mean that t ...
... Reversible reactions • You can recognize that the ammonia-forming reaction reaches a state of chemical equilibrium because its chemical equation is written with a double arrow like this. • At equilibrium, the concentrations of reactants and products are constant. • However, that does not mean that t ...
VOLUME 3 - ICHO 41-45 _opravené_
... Copyright © 2014 by IUVENTA – ICHO International Information Centre, Bratislava, Slovakia You are free to copy, distribute, transmit or adapt this publication or its parts for unlimited teaching purposes, you are obliged, however, to attribute your copies, transmissions or adaptations with a referen ...
... Copyright © 2014 by IUVENTA – ICHO International Information Centre, Bratislava, Slovakia You are free to copy, distribute, transmit or adapt this publication or its parts for unlimited teaching purposes, you are obliged, however, to attribute your copies, transmissions or adaptations with a referen ...
sample chapter
... the COOH group. The double arrow Δ in an equation means that the reaction is reversible; that is, the reaction can occur in both directions. Initially, a number of CH3COOH molecules break up to yield CH3COO⫺ and H⫹ ions. As time goes on, some of the CH3COO⫺ and H⫹ ions recombine to form CH3COOH mol ...
... the COOH group. The double arrow Δ in an equation means that the reaction is reversible; that is, the reaction can occur in both directions. Initially, a number of CH3COOH molecules break up to yield CH3COO⫺ and H⫹ ions. As time goes on, some of the CH3COO⫺ and H⫹ ions recombine to form CH3COOH mol ...
Chapter 5: Gases - HCC Learning Web
... 7. An exothermic reaction causes the surroundings to A) warm up. D) decrease its temperature. B) become acidic. E) release CO2. C) expand. Ans: A Category: Easy Section: 6.2 8. Copper metal has a specific heat of 0.385 J/g·°C. Calculate the amount of heat required to raise the temperature of 22.8 g ...
... 7. An exothermic reaction causes the surroundings to A) warm up. D) decrease its temperature. B) become acidic. E) release CO2. C) expand. Ans: A Category: Easy Section: 6.2 8. Copper metal has a specific heat of 0.385 J/g·°C. Calculate the amount of heat required to raise the temperature of 22.8 g ...
Homework 5-7 answers
... 7. An exothermic reaction causes the surroundings to A) warm up. D) decrease its temperature. B) become acidic. E) release CO2. C) expand. Ans: A Category: Easy Section: 6.2 8. Copper metal has a specific heat of 0.385 J/g·°C. Calculate the amount of heat required to raise the temperature of 22.8 g ...
... 7. An exothermic reaction causes the surroundings to A) warm up. D) decrease its temperature. B) become acidic. E) release CO2. C) expand. Ans: A Category: Easy Section: 6.2 8. Copper metal has a specific heat of 0.385 J/g·°C. Calculate the amount of heat required to raise the temperature of 22.8 g ...
Unit 2: Matter as Solutions and Gases
... Reversible Reactions: - reactions that can proceed forward and in reverse. - when the rate of forward reaction is equalled to the rate of reverse reaction, we say that the process is at equilibrium. (⇌) ...
... Reversible Reactions: - reactions that can proceed forward and in reverse. - when the rate of forward reaction is equalled to the rate of reverse reaction, we say that the process is at equilibrium. (⇌) ...
Chemistry - Department of Education and Skills
... considerable discrepancy in the provision of Physics and Chemistry to girls and boys. Since then provision of these subjects has improved, especially in girls’ schools. However, the most recent analysis of provision indicates the persistence of the problem. Although provision for girls is now best i ...
... considerable discrepancy in the provision of Physics and Chemistry to girls and boys. Since then provision of these subjects has improved, especially in girls’ schools. However, the most recent analysis of provision indicates the persistence of the problem. Although provision for girls is now best i ...
Solutions Manual
... Place hexane and 1-hexene into separate test tubes. Add bromine water to a depth of about 1 cm, shake and allow to settle. If the bromine water is decolourised then the test tube contained 1-hexene and the product of this addition reaction is 1,2-dibromoethane. However, there may also be some 2-brom ...
... Place hexane and 1-hexene into separate test tubes. Add bromine water to a depth of about 1 cm, shake and allow to settle. If the bromine water is decolourised then the test tube contained 1-hexene and the product of this addition reaction is 1,2-dibromoethane. However, there may also be some 2-brom ...
enthalpy changes
... The initial temperature of both solutions was 20°C. The highest temperature reached by the solution was 33°C. Calculate the Molar Enthalpy of Neutralisation. [The specific heat capacity (c) of water is 4.18 kJ oC -1 kg -1] ...
... The initial temperature of both solutions was 20°C. The highest temperature reached by the solution was 33°C. Calculate the Molar Enthalpy of Neutralisation. [The specific heat capacity (c) of water is 4.18 kJ oC -1 kg -1] ...
Part 3-ICHO-31-35
... 3.5 One member of thorium series, after isolation, is found to contain 1.50×1010 atoms of the nuclide and decays at the rate of 3440 disintegrations per minute. What is the half-life in years? ...
... 3.5 One member of thorium series, after isolation, is found to contain 1.50×1010 atoms of the nuclide and decays at the rate of 3440 disintegrations per minute. What is the half-life in years? ...
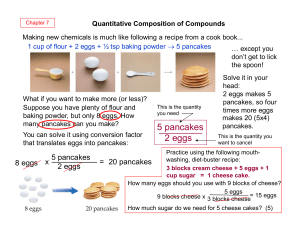
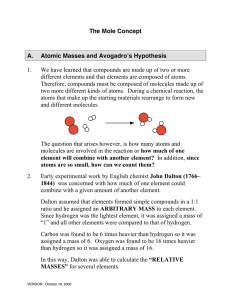
The Mole Concept A. Atomic Masses and Avogadro`s Hypothesis 1
... Dalton assumed that elements formed simple compounds in a 1:1 ratio and he assigned an ARBITRARY MASS to each element. Since hydrogen was the lightest element, it was assigned a mass of “1” and all other elements were compared to that of hydrogen. Carbon was found to be 6 times heavier than hydrogen ...
... Dalton assumed that elements formed simple compounds in a 1:1 ratio and he assigned an ARBITRARY MASS to each element. Since hydrogen was the lightest element, it was assigned a mass of “1” and all other elements were compared to that of hydrogen. Carbon was found to be 6 times heavier than hydrogen ...
full text pdf
... In the milled and thermally activated sorbents further development of the active surface area was observed. Best values were obtained for the electromagnetically milled material (32.8 μm), due to the unique method of milling, leading to increase of material’s reactive properties. SEM microphotograph ...
... In the milled and thermally activated sorbents further development of the active surface area was observed. Best values were obtained for the electromagnetically milled material (32.8 μm), due to the unique method of milling, leading to increase of material’s reactive properties. SEM microphotograph ...
Stoichiometry - AaronFreeman
... Limiting Reactants • The limiting reactant is the reactant present in the smallest stoichiometric amount. – In other words, it’s the reactant you’ll run out of first (in this case, the H2). ...
... Limiting Reactants • The limiting reactant is the reactant present in the smallest stoichiometric amount. – In other words, it’s the reactant you’ll run out of first (in this case, the H2). ...
Mole-Volume Conversion Assignment
... Trial 1: use 1.0g of NaHCO3 and 50mL CH3COOH: extra vinegar left over: NaHCO3 all used Trial 2: use 2.0g of NaHCO3 and 50mL CH3COOH: extra vinegar left over: NaHCO3 all used Trial 3: use 3.5g of NaHCO3 and 50mL CH3COOH: perfect amount of each: both all used up Trial 4: use 4.5g of NaHCO3 and 50mL CH ...
... Trial 1: use 1.0g of NaHCO3 and 50mL CH3COOH: extra vinegar left over: NaHCO3 all used Trial 2: use 2.0g of NaHCO3 and 50mL CH3COOH: extra vinegar left over: NaHCO3 all used Trial 3: use 3.5g of NaHCO3 and 50mL CH3COOH: perfect amount of each: both all used up Trial 4: use 4.5g of NaHCO3 and 50mL CH ...
Homework 5-8 answers
... A) the energy stored within the structural units of chemical substances. B) the energy associated with the random motion of atoms and molecules. C) solar energy, i.e. energy that comes from the sun. D) energy available by virtue of an object's position. Ans: C Category: Easy Section: 6.1 2. Thermal ...
... A) the energy stored within the structural units of chemical substances. B) the energy associated with the random motion of atoms and molecules. C) solar energy, i.e. energy that comes from the sun. D) energy available by virtue of an object's position. Ans: C Category: Easy Section: 6.1 2. Thermal ...
Organic Chemistry Organic Chemistry
... evaporate easily. In fact, they are often gases at room temperature. (b) Polar substances, with strong forces of attraction among the molecules, require considerable energy to evaporate. ...
... evaporate easily. In fact, they are often gases at room temperature. (b) Polar substances, with strong forces of attraction among the molecules, require considerable energy to evaporate. ...
Chapter 18: Chemical Equilibrium
... reaction is spontaneous under standard conditions. Standard conditions are defined as 298 K and one atmosphere pressure. But spontaneous reactions are not always fast. When carried out under standard conditions, this ammonia-forming reaction is much too slow. To produce ammonia at a rate that is pra ...
... reaction is spontaneous under standard conditions. Standard conditions are defined as 298 K and one atmosphere pressure. But spontaneous reactions are not always fast. When carried out under standard conditions, this ammonia-forming reaction is much too slow. To produce ammonia at a rate that is pra ...
Bioorthogonal chemistry
The term bioorthogonal chemistry refers to any chemical reaction that can occur inside of living systems without interfering with native biochemical processes. The term was coined by Carolyn R. Bertozzi in 2003. Since its introduction, the concept of the bioorthogonal reaction has enabled the study of biomolecules such as glycans, proteins, and lipids in real time in living systems without cellular toxicity. A number of chemical ligation strategies have been developed that fulfill the requirements of bioorthogonality, including the 1,3-dipolar cycloaddition between azides and cyclooctynes (also termed copper-free click chemistry), between nitrones and cyclooctynes, oxime/hydrazone formation from aldehydes and ketones, the tetrazine ligation, the isocyanide-based click reaction, and most recently, the quadricyclane ligation.The use of bioorthogonal chemistry typically proceeds in two steps. First, a cellular substrate is modified with a bioorthogonal functional group (chemical reporter) and introduced to the cell; substrates include metabolites, enzyme inhibitors, etc. The chemical reporter must not alter the structure of the substrate dramatically to avoid affecting its bioactivity. Secondly, a probe containing the complementary functional group is introduced to react and label the substrate.Although effective bioorthogonal reactions such as copper-free click chemistry have been developed, development of new reactions continues to generate orthogonal methods for labeling to allow multiple methods of labeling to be used in the same biosystems.