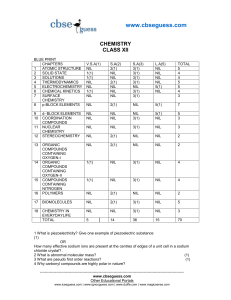
laman web smk raja perempuan, ipoh
... energy changes, principally in the form of heat energy ; the energy changes can be exothermic or endothermic. 2. calculate the heat energy change from experimental measurements using the relationship : energy change = mc∆T 3. define the term enthalphy change of formation, combustion, hydration, solu ...
... energy changes, principally in the form of heat energy ; the energy changes can be exothermic or endothermic. 2. calculate the heat energy change from experimental measurements using the relationship : energy change = mc∆T 3. define the term enthalphy change of formation, combustion, hydration, solu ...
guess paper class xii
... Calculate the mass of a non-volatile solute (molecular mass 40) which should be dissolved in 114 gm octane to reduce its vapour pressure to 80%. 16 In a fuel cell (a device for producing electricity directly from chemical reaction) , methanol is used as fuel and oxygen gas is used as an oxidizer. Th ...
... Calculate the mass of a non-volatile solute (molecular mass 40) which should be dissolved in 114 gm octane to reduce its vapour pressure to 80%. 16 In a fuel cell (a device for producing electricity directly from chemical reaction) , methanol is used as fuel and oxygen gas is used as an oxidizer. Th ...
Predicting Moles of Reactants/Products
... difference between an experimental result and a mathematical result. Using stoichiometry, we can mathematically determine the amount of a product that should be formed during an experiment, yet we sometimes find that we don't end up with exactly the right amount of product. Percentage yield tells us ...
... difference between an experimental result and a mathematical result. Using stoichiometry, we can mathematically determine the amount of a product that should be formed during an experiment, yet we sometimes find that we don't end up with exactly the right amount of product. Percentage yield tells us ...
CHAPTER 18
... Suppose two substances, A and B, react to form products C and D. In turn, C and D react to produce A and B. Under appropriate conditions, equilibrium occurs for this reversible reaction. This hypothetical equilibrium reaction is described by the following general equation. n A + m B $ x C + yD Initi ...
... Suppose two substances, A and B, react to form products C and D. In turn, C and D react to produce A and B. Under appropriate conditions, equilibrium occurs for this reversible reaction. This hypothetical equilibrium reaction is described by the following general equation. n A + m B $ x C + yD Initi ...
Chemistry 11 Final Examination Review
... d) Most of the volume of an atom is empty space. 10. Which of the following orbitals is spherical in shape? a) 3p b) 2s c) 4d d) 5f 11. The third energy level of an atom may have __ electrons. a) 2 b) 18 c) 8 d) 32 12. How many sublevels are possible at the fourth energy level? a) 2 b) 3 c) 4 d) 18 ...
... d) Most of the volume of an atom is empty space. 10. Which of the following orbitals is spherical in shape? a) 3p b) 2s c) 4d d) 5f 11. The third energy level of an atom may have __ electrons. a) 2 b) 18 c) 8 d) 32 12. How many sublevels are possible at the fourth energy level? a) 2 b) 3 c) 4 d) 18 ...
chemistry - Brilliant Public School Sitamarhi
... *12. A compound made up of elements ‘A’ and ‘B’ crystallises in a cubic close packed structure. Atoms A are present on the corners as well as face centres, whereas atoms B are present on the edge-centres as well as body centre. What is the formula of the compound? [Ans. AB] ...
... *12. A compound made up of elements ‘A’ and ‘B’ crystallises in a cubic close packed structure. Atoms A are present on the corners as well as face centres, whereas atoms B are present on the edge-centres as well as body centre. What is the formula of the compound? [Ans. AB] ...
Chemistry - A Quantitative Science
... done the same as converting dozens to items. 1.5 doz = (1.5 doz)(12 items.doz-1) = 18 items and 1.5 mol = (1.5 mol)( 6.0x1023 atoms.mol-1) = 9.0x1023 atoms. The mole is used simply because it is much easier to discuss the number of atoms in moles than it is as individual items - 0.10 mol H2O is a mu ...
... done the same as converting dozens to items. 1.5 doz = (1.5 doz)(12 items.doz-1) = 18 items and 1.5 mol = (1.5 mol)( 6.0x1023 atoms.mol-1) = 9.0x1023 atoms. The mole is used simply because it is much easier to discuss the number of atoms in moles than it is as individual items - 0.10 mol H2O is a mu ...
Version A
... 52. When dilute nitric acid was added to a solution of one of the following chemicals, a gas was evolved, This gas turned a drop of limewater, Ca(OH)2, cloudy, due to the formation of a white precipitate. The chemical was ...
... 52. When dilute nitric acid was added to a solution of one of the following chemicals, a gas was evolved, This gas turned a drop of limewater, Ca(OH)2, cloudy, due to the formation of a white precipitate. The chemical was ...
Part 2-ICHO-26-30
... The overall catalytic reaction is simple, whereas the reaction mechanism in the homogeneous phase is very complicated with a large number of reaction steps, and the course is difficult to control owing to a distinct chain character. With platinum as catalyst the significant reaction steps are: (i) A ...
... The overall catalytic reaction is simple, whereas the reaction mechanism in the homogeneous phase is very complicated with a large number of reaction steps, and the course is difficult to control owing to a distinct chain character. With platinum as catalyst the significant reaction steps are: (i) A ...
Polyamide from lactams by reactive rotational molding via anionic
... 0.2 g/dL with a suspended-level Ubbelohde viscometer at 25°C. Based on the results from the ...
... 0.2 g/dL with a suspended-level Ubbelohde viscometer at 25°C. Based on the results from the ...
Sugar Amino Acids - The Krasavin research group
... during last the two decades to expanding the chemical diversity of this class of hydroxylated cyclic amino acids. Specifically, SAAs have been synthesized mainly as furanoid or pyranoid compounds, and both cyclic and bicyclic scaffolds have been reported (Figure 7.1). There are several advantages to ...
... during last the two decades to expanding the chemical diversity of this class of hydroxylated cyclic amino acids. Specifically, SAAs have been synthesized mainly as furanoid or pyranoid compounds, and both cyclic and bicyclic scaffolds have been reported (Figure 7.1). There are several advantages to ...
17 - Wiley
... 14.43 The pH of an aqueous solution of a salt is determined by the acid–base characteristics of the cation and anion. Because Na+ has no acid–base tendencies, the anions in these compounds determine the pH of their solutions. Solution pH increases with the strength of the basic anion, which in turn ...
... 14.43 The pH of an aqueous solution of a salt is determined by the acid–base characteristics of the cation and anion. Because Na+ has no acid–base tendencies, the anions in these compounds determine the pH of their solutions. Solution pH increases with the strength of the basic anion, which in turn ...
Unit 3 Answer Key
... 3. You would have two times the Avogadro constant of hydrogen atoms. Rounded off, the number would be 2(6.02 × 1023) = 1.20 × 1024 hydrogen atoms. 4. You would not be able to see one person, but a mole of people is so many that they would be visible, as a group, from space. In fact, a mole of pe ...
... 3. You would have two times the Avogadro constant of hydrogen atoms. Rounded off, the number would be 2(6.02 × 1023) = 1.20 × 1024 hydrogen atoms. 4. You would not be able to see one person, but a mole of people is so many that they would be visible, as a group, from space. In fact, a mole of pe ...
Mineralization of Drugs in Aqueous Medium by Advanced Oxidation
... photodiode detector at λ = 210 nm. In these trials a 4 mM H2SO4 solution at 0.6 mL min-1 was employed as mobile phase. Cl− and NO3− concentrations in treated solutions were obtained by ion chromatography using a Shimadzu 10Avp HPLC chromatograph fitted with a Shim-Pack IC-A1S, 10 cm × 4.6 mm, anion ...
... photodiode detector at λ = 210 nm. In these trials a 4 mM H2SO4 solution at 0.6 mL min-1 was employed as mobile phase. Cl− and NO3− concentrations in treated solutions were obtained by ion chromatography using a Shimadzu 10Avp HPLC chromatograph fitted with a Shim-Pack IC-A1S, 10 cm × 4.6 mm, anion ...
Unit 10 complete 2016-2017
... Work the following out on a separate sheet of paper. 1. Hydrogen and oxygen react under certain conditions to product water. a. How many moles of hydrogen would be needed to produce 5.0 moles of water? b. How many moles of oxygen would be needed to produce 5.0 moles of water? 2. Ethane, C2H6, can un ...
... Work the following out on a separate sheet of paper. 1. Hydrogen and oxygen react under certain conditions to product water. a. How many moles of hydrogen would be needed to produce 5.0 moles of water? b. How many moles of oxygen would be needed to produce 5.0 moles of water? 2. Ethane, C2H6, can un ...
Honors Chemistry
... Work the following out on a separate sheet of paper. 1. Hydrogen and oxygen react under certain conditions to product water. a. How many moles of hydrogen would be needed to produce 5.0 moles of water? b. How many moles of oxygen would be needed to produce 5.0 moles of water? 2. Ethane, C2H6, can un ...
... Work the following out on a separate sheet of paper. 1. Hydrogen and oxygen react under certain conditions to product water. a. How many moles of hydrogen would be needed to produce 5.0 moles of water? b. How many moles of oxygen would be needed to produce 5.0 moles of water? 2. Ethane, C2H6, can un ...
OCR AS Level Chemistry B (Salters) H033
... Chemistry B (Salters) was first examined in 1992 as a new concept project examination. In contrast to the traditional ‘topic-based’ approach, Chemistry B (Salters) is ‘context-led’. Chemical concepts are introduced within a relevant context, the course being written as a series of teaching modules b ...
... Chemistry B (Salters) was first examined in 1992 as a new concept project examination. In contrast to the traditional ‘topic-based’ approach, Chemistry B (Salters) is ‘context-led’. Chemical concepts are introduced within a relevant context, the course being written as a series of teaching modules b ...
Chemistry (Revised)
... FeS(s) + 2HCl(aq) → FeCl2(aq) + H2S(g) (i) Hydrogen sulfide gas is very soluble in water. Draw a diagram to show an assembled apparatus that could be used to measure the volume of H2S gas produced when a sample of ...
... FeS(s) + 2HCl(aq) → FeCl2(aq) + H2S(g) (i) Hydrogen sulfide gas is very soluble in water. Draw a diagram to show an assembled apparatus that could be used to measure the volume of H2S gas produced when a sample of ...
CO 2 - TrimbleChemistry
... • A compound is represented by using the symbols for the elements of which it is composed • Subscripts are used to indicate how many atoms of a particular element exist in the compound • If there is only one atom of a particular element, the one is assumed ...
... • A compound is represented by using the symbols for the elements of which it is composed • Subscripts are used to indicate how many atoms of a particular element exist in the compound • If there is only one atom of a particular element, the one is assumed ...
Medicinal Chemistry
... synthetic chemistry molecular modeling computational biology, pharmaceutical chemistry versus medicinal chemistry uf - medicinal chemistry is focused on drug design and chemical synthesis pharmaceutical chemistry also studies drug design and synthesis, medicinal chemistry ms mcphs university - locat ...
... synthetic chemistry molecular modeling computational biology, pharmaceutical chemistry versus medicinal chemistry uf - medicinal chemistry is focused on drug design and chemical synthesis pharmaceutical chemistry also studies drug design and synthesis, medicinal chemistry ms mcphs university - locat ...
Bioorthogonal chemistry
The term bioorthogonal chemistry refers to any chemical reaction that can occur inside of living systems without interfering with native biochemical processes. The term was coined by Carolyn R. Bertozzi in 2003. Since its introduction, the concept of the bioorthogonal reaction has enabled the study of biomolecules such as glycans, proteins, and lipids in real time in living systems without cellular toxicity. A number of chemical ligation strategies have been developed that fulfill the requirements of bioorthogonality, including the 1,3-dipolar cycloaddition between azides and cyclooctynes (also termed copper-free click chemistry), between nitrones and cyclooctynes, oxime/hydrazone formation from aldehydes and ketones, the tetrazine ligation, the isocyanide-based click reaction, and most recently, the quadricyclane ligation.The use of bioorthogonal chemistry typically proceeds in two steps. First, a cellular substrate is modified with a bioorthogonal functional group (chemical reporter) and introduced to the cell; substrates include metabolites, enzyme inhibitors, etc. The chemical reporter must not alter the structure of the substrate dramatically to avoid affecting its bioactivity. Secondly, a probe containing the complementary functional group is introduced to react and label the substrate.Although effective bioorthogonal reactions such as copper-free click chemistry have been developed, development of new reactions continues to generate orthogonal methods for labeling to allow multiple methods of labeling to be used in the same biosystems.