Document
... pizzas, we burn a pizza, drop one on the floor, or other uncontrollable events happen so that we only make two pizzas. The actual amount of product made in a chemical reaction is called the actual yield. We can determine the efficiency of making pizzas by calculating the percentage of the maximum nu ...
... pizzas, we burn a pizza, drop one on the floor, or other uncontrollable events happen so that we only make two pizzas. The actual amount of product made in a chemical reaction is called the actual yield. We can determine the efficiency of making pizzas by calculating the percentage of the maximum nu ...
Document
... group is MORE positive than it was in the C-O-H group By increasing the number of highly electronegative O, more electrons were drawn away from that C, making it more positive. ...
... group is MORE positive than it was in the C-O-H group By increasing the number of highly electronegative O, more electrons were drawn away from that C, making it more positive. ...
Chemistry
... not intend to pursue a major in chemistry). Topics covered include the structure and reactivity of hydrocarbons and functional groups, stereochemistry, aromaticity, nucleophilicity and electrophilicity. Basic types of reactions discussed include nucleophilic substitution, elimination, addition, oxid ...
... not intend to pursue a major in chemistry). Topics covered include the structure and reactivity of hydrocarbons and functional groups, stereochemistry, aromaticity, nucleophilicity and electrophilicity. Basic types of reactions discussed include nucleophilic substitution, elimination, addition, oxid ...
Chemical Reactions - 2012 Book Archive
... antibiotics such as amoxicillin, were unknown only a few years ago. Their development required that chemists understand how substances combine in certain ratios and under specific conditions to produce a new substance with particular properties. ...
... antibiotics such as amoxicillin, were unknown only a few years ago. Their development required that chemists understand how substances combine in certain ratios and under specific conditions to produce a new substance with particular properties. ...
Chapter 4 Chemical Quantities and Aqueous Reactions
... sometimes called the limiting reagent the limiting reactant gets completely consumed ...
... sometimes called the limiting reagent the limiting reactant gets completely consumed ...
File
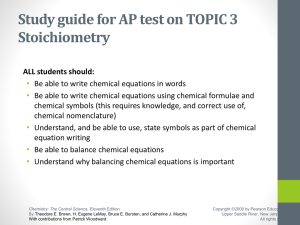
... Take out your stapled packet and complete the left side of the second page (stoichiometry and limiting reagents) Quiz tomorrow will be based on the left side ...
... Take out your stapled packet and complete the left side of the second page (stoichiometry and limiting reagents) Quiz tomorrow will be based on the left side ...
Chapter 4 Chemical Quantities and Aqueous Reactions
... pizzas, we burn a pizza, drop one on the floor, or other uncontrollable events happen so that we only make two pizzas. The actual amount of product made in a chemical reaction is called the actual yield. We can determine the efficiency of making pizzas by calculating the percentage of the maximum nu ...
... pizzas, we burn a pizza, drop one on the floor, or other uncontrollable events happen so that we only make two pizzas. The actual amount of product made in a chemical reaction is called the actual yield. We can determine the efficiency of making pizzas by calculating the percentage of the maximum nu ...
Surface and sub-surface reactions during low temperature
... This study focuses on chemical reactions that occur upon vapor-phase metal-organic precursor exposure during lowtemperature atomic layer deposition on reactive and nonreactive polymer surfaces. Several common polymers with different ...
... This study focuses on chemical reactions that occur upon vapor-phase metal-organic precursor exposure during lowtemperature atomic layer deposition on reactive and nonreactive polymer surfaces. Several common polymers with different ...
Document
... Write balanced equations for the following reactions: (a) The combination reaction that occurs when lithium metal and fluorine gas react. (b) The decomposition reaction that occurs when solid barium carbonate is heated. (Two products form: a solid and a gas.) Solution (a) The symbol for lithium is L ...
... Write balanced equations for the following reactions: (a) The combination reaction that occurs when lithium metal and fluorine gas react. (b) The decomposition reaction that occurs when solid barium carbonate is heated. (Two products form: a solid and a gas.) Solution (a) The symbol for lithium is L ...
General chemistry laboratory activities, Lorentz
... accompanied by their necks, at the ends of which are ground glass joints to quickly and tightly connect to the rest of the apparatus (such as a reflux condenser or dropping funnel). The reaction flask is usually made of thick glass and they can tolerate large pressure differences, with the result th ...
... accompanied by their necks, at the ends of which are ground glass joints to quickly and tightly connect to the rest of the apparatus (such as a reflux condenser or dropping funnel). The reaction flask is usually made of thick glass and they can tolerate large pressure differences, with the result th ...
Holt Modern Chemistry Workbook
... whose simplest units are molecules. In other words, a single molecule of any molecular compound is an individual unit that is capable of existing on its own. A molecule may contain two or more atoms of the same element, as in oxygen. Or, a molecule may consist of two or more atoms of different eleme ...
... whose simplest units are molecules. In other words, a single molecule of any molecular compound is an individual unit that is capable of existing on its own. A molecule may contain two or more atoms of the same element, as in oxygen. Or, a molecule may consist of two or more atoms of different eleme ...
Mechanisms and energetics of surface reactions at the copper
... Due to the strong endergonicity (∆G° >> 0) of the anoxic oxidations 3, 4, and 5, and the very low corresponding equilibrium hydrogen pressures, none of these transformations are expected to be of importance under atmospheric hydrogen pressure. Because of the unusual anoxic conditions and long time-s ...
... Due to the strong endergonicity (∆G° >> 0) of the anoxic oxidations 3, 4, and 5, and the very low corresponding equilibrium hydrogen pressures, none of these transformations are expected to be of importance under atmospheric hydrogen pressure. Because of the unusual anoxic conditions and long time-s ...
AP Chem unit 13 presentation
... It is important to realize that although changes to the reaction may alter the equilibrium positions, they do not alter the equilibrium constant. ...
... It is important to realize that although changes to the reaction may alter the equilibrium positions, they do not alter the equilibrium constant. ...
Document
... 1. The concentrations of the reacting species in the condensed phase are expressed in M. In the gaseous phase, the concentrations can be expressed in M or in atm. 2. The concentrations of pure solids, pure liquids and solvents do not appear in the equilibrium constant expressions. 3. The equilibrium ...
... 1. The concentrations of the reacting species in the condensed phase are expressed in M. In the gaseous phase, the concentrations can be expressed in M or in atm. 2. The concentrations of pure solids, pure liquids and solvents do not appear in the equilibrium constant expressions. 3. The equilibrium ...
- Catalyst
... How to Balance Equations Mass Balance (or Atom Balance)- same number of each element on each side of the equation: (1) start with largest/most complicated molecule (2) progress to other elements, leaving lone elements for last (3) make all whole numbers (4) re-check atom balance 1 CH4 (g) + ...
... How to Balance Equations Mass Balance (or Atom Balance)- same number of each element on each side of the equation: (1) start with largest/most complicated molecule (2) progress to other elements, leaving lone elements for last (3) make all whole numbers (4) re-check atom balance 1 CH4 (g) + ...
AS Chemistry Teacher Handbook
... adverse consequences for living cells of exposure to radiation and use of radioisotopes in many contexts, including health, medicine, radio-dating, industry and analysis ...
... adverse consequences for living cells of exposure to radiation and use of radioisotopes in many contexts, including health, medicine, radio-dating, industry and analysis ...
mcdonald (pam78654) – HW 1: High School Concepts – laude
... In this case, all of these numbers are whole numbers or are close enough for rounding to a whole number. The empirical formula is C3 H3 O. Next we find the molecular formula. The molecular formula gives the actual number of atoms of each element present in a molecule of the compound. We were given t ...
... In this case, all of these numbers are whole numbers or are close enough for rounding to a whole number. The empirical formula is C3 H3 O. Next we find the molecular formula. The molecular formula gives the actual number of atoms of each element present in a molecule of the compound. We were given t ...
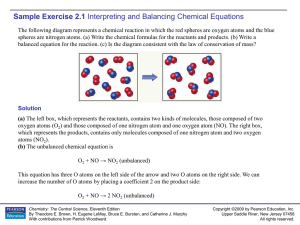
Sample Exercise 2.1
... Write balanced equations for the following reactions: (a) The combination reaction that occurs when lithium metal and fluorine gas react. (b) The decomposition reaction that occurs when solid barium carbonate is heated. (Two products form: a solid and a gas.) Solution (a) The symbol for lithium is L ...
... Write balanced equations for the following reactions: (a) The combination reaction that occurs when lithium metal and fluorine gas react. (b) The decomposition reaction that occurs when solid barium carbonate is heated. (Two products form: a solid and a gas.) Solution (a) The symbol for lithium is L ...
Stoichiometry Chapter 3 CHEMA1301 [Compatibility Mode]
... Na2CO3 + 2HCl g 2NaCl + H2O + CO2 We have one mole of Na2CO3 and two moles of HCl, therefore, can write: No. of moles Na2CO3 = ½ * No. of moles HCl No. of moles HCl = 2 * No. of moles Na2CO3 Also from mole relationships in the balanced equation, we can formulate the following: mol Na2CO3 = mol H2O m ...
... Na2CO3 + 2HCl g 2NaCl + H2O + CO2 We have one mole of Na2CO3 and two moles of HCl, therefore, can write: No. of moles Na2CO3 = ½ * No. of moles HCl No. of moles HCl = 2 * No. of moles Na2CO3 Also from mole relationships in the balanced equation, we can formulate the following: mol Na2CO3 = mol H2O m ...
Bioorthogonal chemistry
The term bioorthogonal chemistry refers to any chemical reaction that can occur inside of living systems without interfering with native biochemical processes. The term was coined by Carolyn R. Bertozzi in 2003. Since its introduction, the concept of the bioorthogonal reaction has enabled the study of biomolecules such as glycans, proteins, and lipids in real time in living systems without cellular toxicity. A number of chemical ligation strategies have been developed that fulfill the requirements of bioorthogonality, including the 1,3-dipolar cycloaddition between azides and cyclooctynes (also termed copper-free click chemistry), between nitrones and cyclooctynes, oxime/hydrazone formation from aldehydes and ketones, the tetrazine ligation, the isocyanide-based click reaction, and most recently, the quadricyclane ligation.The use of bioorthogonal chemistry typically proceeds in two steps. First, a cellular substrate is modified with a bioorthogonal functional group (chemical reporter) and introduced to the cell; substrates include metabolites, enzyme inhibitors, etc. The chemical reporter must not alter the structure of the substrate dramatically to avoid affecting its bioactivity. Secondly, a probe containing the complementary functional group is introduced to react and label the substrate.Although effective bioorthogonal reactions such as copper-free click chemistry have been developed, development of new reactions continues to generate orthogonal methods for labeling to allow multiple methods of labeling to be used in the same biosystems.
















![Stoichiometry Chapter 3 CHEMA1301 [Compatibility Mode]](http://s1.studyres.com/store/data/014247793_1-84b4b6fe6fa37d77afbf7eb657ee347a-300x300.png)