* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download BASIC CONCEPTS OF CHEMISTRY
Computational chemistry wikipedia , lookup
Bond valence method wikipedia , lookup
Radical (chemistry) wikipedia , lookup
Marcus theory wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
Atomic orbital wikipedia , lookup
Electronegativity wikipedia , lookup
Metastable inner-shell molecular state wikipedia , lookup
Isotopic labeling wikipedia , lookup
Electrolysis of water wikipedia , lookup
Bent's rule wikipedia , lookup
Acid dissociation constant wikipedia , lookup
Molecular orbital diagram wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Photoredox catalysis wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Click chemistry wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Stoichiometry wikipedia , lookup
History of chemistry wikipedia , lookup
Metallic bonding wikipedia , lookup
Resonance (chemistry) wikipedia , lookup
Transition state theory wikipedia , lookup
Inorganic chemistry wikipedia , lookup
Coordination complex wikipedia , lookup
Equilibrium chemistry wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Molecular dynamics wikipedia , lookup
Organosulfur compounds wikipedia , lookup
Stability constants of complexes wikipedia , lookup
Electrochemistry wikipedia , lookup
Organic chemistry wikipedia , lookup
Acid–base reaction wikipedia , lookup
Chemical reaction wikipedia , lookup
Electron configuration wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Biochemistry wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Atomic theory wikipedia , lookup
Chemical bond wikipedia , lookup
Metalloprotein wikipedia , lookup
Olga V. Kovalchukova, Nasrin Namichemazi
& Rusul Alabada
Lectures in
CHEMISTRY
For the 1-st year students of the «Dentistry»
speciality of the Medical Faculty of the Peoples’
Friendship University of Russia
Ковальчукова О.В., Насрин Намичемази,
Русул Алабада
ХИМИЯ
(конспект лекций)
Для студентов 1 курса медицинского
факультета специальности «Стоматология»
(на английском языке)
Москва
Издательство Российского университета дружбы народов, 2015
Утверждено
РИС Ученого совета
Российского университета дружбы народов
Ковальчукова О.В., Насрин Намичемази, Русул Алабада.
Химия. Конспект лекций для студентов I курса медицинского
факультета специальности «Стоматология». М.: Изд-во РУДН, 2015. –
164 с.
Настоящее учебное пособие представляет собой конспект
лекций, предназначенный для студентов медицинского факультета
специальности «Стоматология». Составлено в соответствии с
Федеральным государственным образовательным стандартом и
программой курса «Химия».
Предназначено для работы студентов I курса медицинского
факультета специальности «Стоматология» при подготовке к
лабораторным занятиям и к экзамену по курсу «Химия».
Подготовлено на кафедре общей химии.
The manual represents the lecture book of the Chemistry discipline
delivering for the students of the «Dentistry» speciality of the Medical
Faculty of Peoples’ Friendship University of Russia. It is prepared in
accordance with the Federal state educational standard and the Program in
Chemistry.
Intended for the 1-st year students of the «Dentistry» speciality of
the Medical Faculty of the Peoples’ Friendship University of Russia.
Prepared at the department of General Chemistry.
© Ковальчукова О.В., Насрин Намичемази, Русул Алабада
© Издательство Российского университета дружбы народов, 2015
2
SUMMARY
Page
Part 1. General Chemistry
Introduction……………………………………………………….
General notions of Chemistry……………………………………
General Laws of Chemistry……………………………………...
Classes of Inorganic Compounds………………………………..
Concepts of Chemical Thermodynamics…………………………
Chemical Kinetics….…………………………………………….
Structure of Atoms………………………………………………
Periodic Law and Periodic System of Chemical Elements……….
Chemical bonds……………………………………………………
Adsorption…………………………………………..………….….
Concepts of Catalysis………………………………………….…..
Solutions………………………………………..…………………
Physico-Chemical Processes in solutions…………..…………….
Dissociation of Strong Electrolytes…...…………………………
Dissociation of weak electrolytes………………………………….
Electrolytic Dissociation of Water………………………………
Equilibria in Solutions with Precipitates..……………………...
Buffer solutions………………………………………………….
Direction of reactions of ionic exchange…………………………
Hydrolysis of salts…………………………………………………
Colloidal solutions………………………………………………..
Oxidation-reduction reaction (redox-reactions)…………………
Complex compounds……………………………………………..
3
5
7
9
15
18
21
28
30
40
43
45
46
49
50
51
52
54
57
59
64
70
76
Part 2. Inorganic Chemistry
s-Elements (alkaline and alkaline earth metals)…………………
Elements of IIIA and IVA groups (p-elements)………………….
Elements of VA and VIA groups (p-elements)………………….
Elements of the VIIA group (halogens)…………………………..
Transitional elements (d-elements)……………………………….
85
87
89
90
92
3
Electrochemical corrosion of metals……………………………..
93
Part 3. Organic Chemistry
The theory of chemical structure of organic compounds………...
Alkanes…………………………………………………………….
Cycloalkanes………………………………………………………
Alkenes…………………………………………………………….
Alkadienes…………………………………………………………
Alkynes…………………………………………………………….
Aromatic hydrocarbons…………………………………………...
Organic compounds with functional groups……………………..
Amines…………………………………………………………….
Alcohols……………………………………………………………
Phenols……………………………………………………………
Aldehydes and ketones…………………………………………….
Carboxylic acids…………………………………………………...
Aminoacids and proteins…………………………………………
Esters, fats and oils……………………………………………….
Carbohydrates…………………………………………………….
Sulphur in organic compounds………………………………….
Biologically important heterocyclic compounds…………………
List of citated literature………………………………………….
4
97
106
108
108
110
112
113
118
119
122
128
130
135
142
145
148
156
157
164
121
121
125
128
131
136
142
146
149
157
157
PART 1
GENERAL CHEMISTRY
5
6
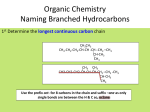
BASIC CONCEPTS OF CHEMISTRY
Objects of studying of chemistry are substances and their smallest
particles - molecules and atoms.
Molecule is the least particle of substance possessing its chemical
properties.
Atom is the least particle of a chemical element possessing its
chemical properties. Atoms are components of molecules.
Chemical element is the kind of atoms characterized by certain set
of properties.
Mole is a unit of measurement of quantity of substances, containing
such amount of molecules, atoms or other structural units, as 12 g of an
isotope 12С.
The number of structural units containing in 1 mole of substances,
is known as Avogadro’s number (NA): NA = 6,021023 mol-1.
The mass of 1 mole of a substance expressed in grams, is called a
molar mass of a substance (M, g x mol-1).
Quantity of a substance:
= m / M = N / NA (mole).
GENERAL LAWS OF CHEMISTRY
1. Incorporated gas law:
PV P0 V0
T
T0 .
2. Avogadro’s Law: equal volumes of various gases under
identical conditions (temperature and pressure) contain the
identical number of molecules.
Consequence 1. Masses of two identical volumes of various gases
under identical conditions concern as their molar masses:
7
m1 M 1
D
m2 M2
(relative density of a gas)..
Consequence 2. Under normal conditions (Р0 = 101325 Pa and Т0
= 273 K) one mole of any gas occupies the volume of 22,4 l (VМ - molar
volume).
3. Mendeleyev-Klayperon’s equation:
PV
m
RT
M
, where R - a universal gas constant (R = 8,314 J x
mole-1 x K).
4. Partial pressure of gas is the part of a total pressure of a gas
mixture which is necessary on a share of the given gas.
According to Dalton’s law, partial pressure of gas in a mixture
equals the pressure of gas as if it occupied the total volume
under the same temperature.
Рtot. = Р1 + Р2 + … + Рn.
5. The law of mass conservation. Sum of masses of the
substances entering a chemical reaction, equal total mass of the
products.
6. The law of equivalents. Substances react with each other in
the quantities proportional to their equivalents:
n1 = n2 (n - number of equivalents).
The equivalent is such a quantity of substance which reacts with 1
mole of hydrogen atoms or replaces them in chemical reactions (the
quantity of substance corresponding to a unit valency).
Equivalent mass (МE) is the mass of one equivalent of the
substance, expressed in grams:
МE = f x M (where f is the factor of equivalence).
8
Calculation of the factor of equivalence of different classes of
inorganic compounds:
For simple substances and elements in chemical compounds
f = 1 / V (where V is the valency of an element).
For acids and the bases
f = 1 / m (where m is the basidity of an acid or acidity of a
base).
For oxides and salts
f = 1 / n x V (where n is the number of metallic atoms in the
compound, and V is the valency of the metal).
Number of equivalents: n = m / МE (for any substance); n = V / VE
(for gaseous substances), VE is the equivalent volume of the gas (the
volume occupied by one equivalent of a gas under normal conditions). For
example, under normal conditions the equivalent volume of hydrogen (МE
= 1 g x mole-1) equals 11,2 liters, and equivalent volume of oxygen (МE = 8
g x mole-1) equals 5,6 litres.
GENERAL CLASSES OF INORGANIC COMPOUNDS
OXIDES
Oxides are binary compounds containing oxygen atoms in the –2
oxidation state.
9
OXIDES
Salt-forming
Not salt-forming
(indifferent)
Basic
Amphoteric
Acidic
Metallic
oxides in
low
oxidation
states
(+1, +2)
Some metallic
oxides in the
oxidation states
+2, +3 and +4
Non-metallic
oxides in oxidation
states +3 and
above, as far as
metallic oxides in
oxidation states +4
and above
Non-metallic
oxides in
oxidation states
+1 and+2
Na2O, Cu2O,
FeO, MgO
BeO, ZnO,
PbO, SnO,
Al2O3, Cr2O3,
MnO2
В2О3, СО2, P2O5,
PbO2, CrO3
СО, SiO, N2O,
NO
Increase in oxidation state of a non-metal changes properties of its
oxide from indifferent to acidic:
N2O
NO
N2O3
NO2
N2O5
Indifferent oxides
Acidic oxides
Increase in oxidation state of a metal changes properties of its
oxide from basic via amphoteric to acidic:
MnO
Mn2O3
MnO2
MnO3
Mn2O7
Basic oxides
Amphoteric
Acidic oxides
oxide
Chemical properties of oxides:
10
1. Basic and acidic oxides are dissolved in water (react with it) in case
if soluble bases and acids are formed::
Na2O + H2O = 2NaOH
Only basic oxides of alkaline and
CaO + H2O = Ca(OH)2
alkaline-earth metals
FeO + H2O
SO3 + H2O = H2SO4
P2O5 + H2O (хол.) = 2НРО3
P2O5 + 3H2O (гор.) = 2Н3РО4
2NO2 + H2O = HNO2 + HNO3
4NO2 + O2 + 2H2O = 4HNO3
СrO3 + H2O = H2CrO4
SiO2 + H2O
Only acidic oxides which form
soluble acids
2. Acidic oxides react with basic oxides:
Na2O + СО2 = Na2СО3
If the acidic oxide is in the
gaseous phase, the reaction takes
CaO + SO2 = CaSO3
place under room temperature
melting
If the acidic oxide is solid, the
Na2O + SiO2 Na2SiO3
reaction takes place undervhigh
melting
temperatures (melting)
FeO + PbO2 FePbO3
3. Amphoteric oxides can react with both acidic and basic oxides:
ZnO + Na2O Na2ZnO2
ZnO + CO2 = ZnCO3
melting
melting
2KAlO2
melting
Al2O3+3SiO2 Al2(SiO3)3
Al2O3 + K2O
11
BASES
Bases are substances, which form hydroxyl-anions (OH-) at
dissociation.
Number of OH-groups of the base defines its acidity.
Chemical properties of bases:
Alkalis:
1) react with acidic oxides and acids:
2КОН + SO3 = K2SO4 + H2O
Ca(OH)2 + 2HCl = CaCl2 + 2H2O
Reaction of
neutralization
2) react with salts (in case if a precipitate is formed):
2NaOH + FeCl2 = Fe(OH)2 + 2NaCl
Ba(OH)2 + Na2SO4 = BaSO4 + 2NaOH
NaOH + BaCl2
3) react with amphoteric oxides and amphoteric bases:
2NaOH + BeO
3NaOH + Cr(OH)3
melting
Na2BeO2 + H2O
solution
Na3[Cr(OH)6]
4) dissociate in aqueous solutions and change the colour of acidbase indicators:
NaOH = Na+ + OH–
(methylorange turns yellow, lithmuth turns blue, and phenolphtalein turns
pink)
12
Insoluble bases:
1) react with acidic oxides and acids (reaction of neutralization):
Fe(OH)3 + 3HNO3 = Fe(NO3)3 + 3H2O
t
2) decompose at heating: Cu(OH)2
CuO + H2O
Amphoteric bases (bases which correspond to amphoteric oxides):
1) react with acidic oxides and acids (reaction of neutralization):
Zn(OH)2 + CO2 = ZnCO3 + H2O
Al(OH)3 + 3HCl = AlCl3 + 3H2O
2) react with alkalis:
Sn(OH)2 + 2NaOH = Na2[Sn(OH)4]
3) decompose at heating:
t
2Al(OH)3
Al2O3 + 3H2O
ACIDS
Acids are substances, which form hydrogen kations (H+) at
dissociation.
Basicity of an acid (number of H-atoms) defines the possibility of
full or incomplete neutralisation of an acid in reactions with bases:
HCl + NaOH = NaCl + H2O
(for monobasic acids only one reaction of neutralisation is possible).
For the multibasic acids full and incomplete neutralisation is
possible: H2SО4 + 2КОН = К2SО4 + 2Н2О (full neutralisation)
H2SО4 + КОН = КНSО4 + Н2О (incomplete neutralization)
13
Chemical properties of acids:
1.
2.
3.
Change in colour of indicators (methylorange and lithmus turn red).
Interaction with active metals: Са + 2HCl = CaCl2 + H2
Interaction with basic and amphoteric oxides:
СuO + 2HNO3 = Cu(NO3)2 + H2O
Al2O3 + 3H2SO4 = Al2(SO4)3 + 3H2O
4.
Interaction with bases (reaction of neutralization):
Cu(OH)2 + 2HCl = CuCl2 + 2H2O
5.
Interaction with salts (reactions of ionic exchange):
H2SO4 + BaCl2 = 2HCl + BaSO4 (precipitate is formed)
2HNO3 + СаСО3 = Ca(NO3)2 + H2O + CO2 (gas is evolved)
HCl + KNO2 = KCl + HNO2 (weak acid is formed)
SALTS
Salts are substances, which form metallic kations or ammonium
(NH4+) and acidic anions at dissociation.
Salts can be considered as products of replacement of hydrogen
atoms in an acid molecule by metals or hydroxyl-groups in a base by an
acidic anion.
Chemical properties of salts:
1.
2.
3.
14
Interaction with metals (a more active metal replaces a less active
one):
FeCl2 + Zn = Fe + ZnCl2.
Interaction with non-metals (a more active non-metal replaces a less
active one):
2NaBr + Сl2 = Br2 + 2NaCl.
Interaction with alkalis (reactions of ionic exchange):
MgCl2 + 2NaOH (exsess) = Mg(OH)2 + 2NaCl.
MgCl2 + NaOH (lack) = MgOHCl + NaCl.
4.
Interaction with acids (reactions of ionic exchange):
СаCl2 + H2SO4 (lack) = CaSO4 + 2HCl.
CaSO4 + H2SO4 (exsess) = Ca(HSO4)2
5.
Interaction between two salts (reactions of ionic exchange):
CuCl2 + 2AgNO3 = 2AgCl + Cu(NO3)2.
CONCEPTS OF CHEMICAL THERMODYNAMICS
The study of chemical processes should be approached through a
series of successive approximations. The first stage is expedient to consider
only the initial and final states of the interacting bodies without considering
the way in which the process takes place, and the development of the
process in time. This approach is called the thermodynamics. For
convenience, the objects of study should be isolated. Such a collection of
bodies extracted from the space, is called the system . If no mass and heat
transfer exists between the system and the surrounding environment, the
system is called isolated. If this condition is not met, then the system is
called open. If the system is only possible for the heat transfer , it is called
closed.
A state of any system is characterized by certain thermodynamic
parameters , which include temperature (T ) , pressure ( P) , volume (V),
chemical composition . The change of at least one of the parameters leads
to a change in the system state.
State of the system can be represented in the form of so-called state
equation:
( P , V, T) = 0
For majority of systems, the state equations are empirical , that is,
the experimentally obtained equations describing the behavior of matter in
a certain range of pressure and temperature.
15
For the thermodynamic description of the system, the state
functions are used. These are equations that can be uniquely identified by
the parameters P, V and T. The values of these functions are independent of
the nature of the process, resulting in a system of this state . The functions
of the state are: 1) the internal energy of the system (U);
2) the enthalpy ( heat content ) of ( H) ;
3 ) the entropy (a measure of disorder) of the system (S);
4) the Gibbs’ free energy (G);
5) the Helmholtz’s free energy (F).
Chemical reactions are accompanied by the release or absorption of
energy usually in the form of heat. Th reactions in which heat is released
are called exothermic reactions and extending the absorption of heat endothermic. Since the heat generation decreases the enthalpy of the
system, then Q = - H, where Q is the heat of the reaction, and H is the
change in enthalpy of the system.
Thus, the condition of the exothermic reaction is Q> 0 or H < 0,
and the condition of the endothermic reaction - Q < 0 or H> 0 .
The equation of a chemical reaction involving the magnitude of the
thermal effect (enthalpy) is called a thermochemical equation:
2H2 ( g ) + O2 ( g ) = 2H2O ( g) + 571.6 kJ or
2H2 ( g ) + O2 ( g ) = 2H2O ( g) ; H = 571.6 kJ
Heat of formation of compounds is the amount of heat released
during the formation of 1 mole of the compound from elements in their
most stable modification . Thus, the heat of formation of water
Hf (H2O ) = 571.6 / 2 = 285.8 kJ / mol
The heat of formation of the substance, measured in standard
conditions (T = 298 K, P = 101325 Pa), is called the standard heat of
formation , and is denoted H0. The standard heat of formation of simple
substance in its most stable modification shall be equal to zero.
Calculation of the heat of the reaction from the heats of formation
of the participating substances, is produced by the Hess’ law , the heat of
the chemical reaction depends on the starting and final products state and is
not dependent on any stage after the reaction proceeds . The thermal effect
16
of the process is the sum of the thermal effects of the individual stages of
the process.
For example, coal combustion may take place in a single step:
C (s) + O2 (g) = CO2 (g); H1 = 395.4 kJ
or through the intermediate formation of carbon oxide (II):
a) C (s) + 1 / 2O2 (g) = CO (g); H2 = 110.7 kJ
b) CO (g) + 1 / 2O2 (g) = CO2 (g); H3 = 284.7 kJ .
The total heat released by the reaction in both cases is the same:
H1 = H2 + H3.
By the corollary of the Hess’ law, the heat of the reaction is equal
to the difference between the sum of the standard heats of formation of the
final products and raw materials. For example, the reaction
MgO (s) + CO2 (g) = MgCO3 (s)
H0 reaction = H0 (MgCO3) - [H0 (MgO) + H0 (CO2)] =
= 115.6 - (- 602.0 - 395.4 ) = 1113.0 kJ .
The standard heats of formation of substances are tabulated data.
There are two driving forces for the nature of the processes
occurring in the nature. These are the desire to move to the lower energy
state (H < 0) and the desire to move into a state of the greatest disorder
(entropy) (S> 0). Since chemical reactions usually proceed with the change
of the energy of the system and its entropy, then the reaction’s direction
that where the total driving force of the reaction decreases. Under the
isobaric- isothermal conditions (pressure and temperature do not change)
the total driving force of the reaction is called the Gibbs’ energy:
G = H - TS
The negative value of the Gibbs’ energy change (G < 0) is the
condition for the spontaneous reaction.
The temperature at which G = 0, is called the reaction initiation
temperature. In this case
TG = 0 = H / S.
17
Changes in the Gibbs’ energy and entropy in chemical reactions are
similar to the change in the enthalpy (heat effect) and determined in
accordance with the result of the Hess’ law:
H0 = (H0products - H0 . reactants)
G0 = (G0products - G0 . reactants)
S0 = (S0products - S0. reactants) .
CHEMICAL KINETICS
One of the basic concepts of chemical kinetics is the concept of a
rate of a chemical reaction.
Rate of a chemical reaction is denoted as number of elementary
acts of a reaction which results transformation of reactants into reaction
products, in a unit time in a unit volume.
In practice, rates of reactions can be measured as a change in
concentrations of substances participating in it for a certain time interval:
v
c
Out of two chemical reactions, that one is of the greatest rate, in
which under identical time more quantity of a substance is formed.
Law of mass action. Collision of molecules should be a necessary
condition for realisation of chemical interaction between molecules.
Collision occurs the more often, than more molecules contains in the given
volume, i.e. rate of a chemical reaction depends on concentrations of
reacting substances.
aA bB mM nN
v k A B
where k is a rate constant of the chemical reaction, numerically it equals
rate of a reaction at unit concentrations of reacting substances.
, - simple numbers, usually not more than 3. For simple
reactions they correspond to stoitiometric coefficients of the reaction.
18
The rate of the reaction does not depend on concentrations of firm
substances, but only on their surface area.
CaO + CO2
CaCO3
v k CO2
v k 2
The equation of a reaction frequently does not reflect its
mechanism. For example, the reaction
2HI + H2O2 2H2O + I2
really proceeds in two stages:
1. HI + H2O2 HOI + H2O (slowly)
2. HOI + HI I2 + H2O (quickly)
Kinetics of the overall reaction it is described by the first (slow)
stage. Expression of speed of this reaction registers as
v k HI H 2 O2
instead of
,
v k HI H 2 O2
2
Temperature dependence of rates of chemical reactions. Rate
of a chemical reaction depends on number of effective collisions. Effective
collision occurs only between active molecules. Increase in temperature
increases number of active molecules, providing them with necessary
activation energy, and the rate of the reaction increases.
Activation energy is that additional energy which it is necessary to
transfer to system to start chemical reaction.
Vant Hoff’s rule. At increase in temperature on 10 speed of
reaction increases in 2 - 4 times.
T2 T1
v 2 v1 10 ,
v2
v1
where
is the rate of a reaction at temperature T2,
is the rate of a reaction at temperature T1,
is the temperature coefficient of the reaction which defines change of
the rate of the reaction at temperature change on 10.
19
v
As
the reactional time.
c
T2 T1
1 2 10 , where
so
is
Chemical equilibrium
If a chemical reaction can proceed only in one direction it is called
as irreversible. The reactions proceeding simultaneously in two directions,
are reversible. Eventually rate of a direct reaction (v ) decreases, and rate
of a back reaction (v ) increases until they become equal. So, a chemical
equilibrium is established in the system. The condition of a chemical
equilibrium:
v = v .
In the equilibrium state, reversible reactions are described by an
equilibrium constant K:
aA + bB
K
c
C
a
A
C C
C C
d
D
b
B
cC + dD
where
CA, CB, CC, CD are concentrations of gaseous or dissolved substances.
Chemical equilibrium is a dynamic one, so it can be shifted
according to le Chateleu’s principle (principle of counteraction): if an
equilibrium system is affected by any factor (change in concentrations,
pressure or temperature), the equilibrium will be shifted in the direction
which weakens the external influence.
The increase in temperature shifts the equilibrium towards an
endothermic reaction (the system absorbs heat and increases its internal
energy, H>0), and decrease in temperature shifts the equilibrium towards
20
an exothermic reaction (the system evolves heat and decreases its internal
energy, H <0).
The increase in pressure causes shifting of the equilibrium towards
less quantity of gaseous substances (as pressure is affected only by gaseous
substances), and decrease in pressure shifts the equilibrium towards more
quantity of gaseous substances. In case if quantities of gaseous substances
among reactants and products are same, change in pressure does not cause
shifting of chemical equilibrium.
The increase in concentration of one of reactants causes shifting of
equilibrium towards formation of products of reaction, and increase in
concentration of one of reactional products shifts the equilibrium towards
reactants.
STRUCTURE OF ATOMS
Atom is a complicated particle consisting of positively charged
atomic nucleus and electronic shells where negatively charged electrons are
located.
The positive charge of a nucleus equals the sum of negative
charges of electrons.
The nucleus itself consists of positively charged protons and
uncharged neutrons. Protons, neutrons and электроны carry the name
«elementary particles».
Using relative units, one can state that electrons are –1 charged,
and protons are +1 charged.
The mass of an atom expressed in nuclear mass units, is called a
relative nuclear mass or mass number of an atom, Мr. It equals sum of
masses of all elementary particles of the atom. As mass numbers of
protons and neutrons are equal (1 a.m.u.), and masses of electrons are
neglectably small (approximately 2,000 times less than corresponding
masses of protons and neutrons) mass number of an atom equals sum of
number of protons and neutrons.
21
Symbols of chemical elements are usually represented in the way:
a X c
b
,
where X is the symbol of the element;
a is the mass number of the element;
b
is an element serial number in the Periodic Table (equals
number of protons in the atom);
c is the charge of the ion.
Natural chemical elements exist in the form of a mixture of
isotopes.
Isotopes are atoms of the same chemical element with identical
number of protons, but different mass numbers (number of neutrons). For
35
example, natural chlorine exists in the form of two isotopes: 17
37
17
Cl
(17
Cl
protons and 18 neutrons), and
(17 protons and 20 neutrons). Relative
masses of elements in periodic system of elements, are average masses of
natural isotopes.
Structure of electronic shells
Electrons possess so called wawe dualism (simultaneously
properties of a particle and a wave).
In this connection, for the description of properties электрона enter
special function which name state function of an electron or wave
function, . It is entered in such a manner that the square of its module is
proportional to probability to find out a particle (electron) in the given
place at the appointed time (probability density).
Wave function of an electron is called «orbital». It characterises
energy and the form of spatial distribution of an electronic cloud.
22
Quantitative parities in the theory of a structure of atom are defined
by the Shrodinger’s wave equation:
h 2 2 2 2
U E
8 2 m x 2 y 2 z 2
where U is potential energy of the electron;
Е is full energy of the electron;
m is the mass of the electron;
x, y, z are spacial co-ordinates of the electron;
is the wave function;
Consequence of the decision of Shrodinger’s equation
Шредингера is the set of four quantum numbers which characterise
behaviour of the electron in the atom.
n, principle quantum number, it defines the general stock of
energy of the electron, i.e.energetic shell.
n = 1,2,3 …
l, azimutal quantum number, defines the form of electronic
orbital (subshell).
l = 0,1,2 … (n-1).
If l = 0 the orbital is called s-orbital (spheric movement of the
electron). At l = 1 we have p-orbital (double-lobe movement form).
Movement forms of d - and f - orbitals (l = 2 and 3 accordingly) have even
more complicated character.
The number of orbitals at an energetic level coincides with its
number. So, for the first shell (n = 1) there is only one subshell (l = 0), that
is 1s-orbital. Similarly for n = 2 (the second shelll) two subshells exist (l =
0, 1) or 2s, 2p-orbitals; for the third shell (n = 3, l = 0, 1, 2) 3s, 3p, and 3dorbitali exist etc.
ml is the magnetic quantum number, it characterises projection
of the magnetic moment of the electron on an external magnetic field, that
is defines the spacial orientation of the electronic orbital. Its values are
defined by azimutal quantum number:
ml = l; (l-1); (l-2) … 0
23
For the orbital quantum number l = 0, magnetic quantum number
has one possible value (ml = 0), that is only one way of orientation of sorbital in space is possible. Similarly we receive, that for p-orbitals (l = 1,
ml =-1, 0, +1) there are three possible ways of orientation (along coordinate axes), for d-orbitals there are five possible ways of orientation (l =
2, ml =-2,-1, 0, +1, +2).
To specify the concept electronic orbital, we can state that it
represents a set of positions of electrons in the atom. Conditionally nuclear
orbitals are designated in the form of cages (energetical cells):
1s
2s
2p
3s
3p
3d
ms , the spin quantum number, defines the moment of spinning
of the electron. As there are only two ways of spinning (clockwise and
anticlockwise), the magnetic quantum number can accept two values:
ms = l.
Conditionally, electrons having different values of spin quantum
number, are designated by opposite directed arrows: .
Electronic formulae of atoms
If the atom is in the ground state (does not possess superfluous
energy) its electrons occupy the lowest energetic orbitals. Energy of an
electron in multielectronic atoms depends not only on its attraction to a
nucleus, but also from repultion from other electrons. Mutual influence
leads to that energy of the electron depends not only on principle, but also
on azimutal quantum number.
24
Klechkovsky’s rules
1. The increase in energy of electronic subshells goes as increase in
the sum of the principle and azimutal quantum numbers (n+l).
2. In case of equality of the sum (n+l) the increase in energy of
subshell goes as increase in the principle quantum number.
Graphically it is possible to present the Klechkovsky’s rule in a
kind:
n
1
2
3
4
5
l
0
1s
2s
3s
4s
5s
1
2
3
2p
3p
4p
5p
3d
4d
5d
4f
5f
Filling of orbitals by electrons occurs in a following order: 1s, 2s,
2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p etc.
Pauli's exclusive principle
In one atom, there cannot be two electrons with an identical set of
quantum numbers. Because everyone electronic orbital is characterised by a
set of three quantum numbers (principle, azimutal and magnetic), electrons
of the same orbital can differ only by the value of spin quantum number (ms
= ). A consequence of a Pauli’s principle of is that one orbital can
contain not more than two electrons.
In connection with the previously mentioned at the first energetic
shell, not more than two electrons can exist:
Or 1s2;
1s
And the second energetic shell can maximally contain 8 electrons:
Or 2s22p6 etc.
2s
2p
The maximum number of electrons at any shell N = 2n2, where n
is the principle quantum number.
25
Hund’s rule
In a subshell electrons fill orbitals so that the total spin quantum
number becomes maximum (orbitals of a subshell are first filled by one
electron each and only after all orbitals are filled, pairing of electrons takes
place).
For example, four electrons on a p-subshell can be arranged in two
different ways:
Or
(ms) = + 1
(ms) = 0
As in the first case the total spin number is more,the first electronic
structure is realized.
Electronic formulae of atoms and ions
The number of electrons in atom is defined by an element serial
number in the Periodic table. Using the above rules and principles, for a
sodium atom of (11 electrons) the following electronic formula is received:
2 2
6 1
11Na: 1s 2s 2p 3s
1s
2s
2p
3s
The electronic formula of a Ti atom:
2 2
6 2
6 2
2
22Ti: 1s 2s 2p 3s 3p 4s 3d
1s
2s
4s
3d
2p
3s
3p
If one electron do not suffice to full or half-full d-subshell (d10 or
d -configurations), it is transmitted from the next s-subshell. As a result, the
electronic formula of Cr atom looks like 24Cr: 1s22s22p63s23p64s13d5,
5
26
instead of 24Cr: 1s22s22p63s23p64s23d4, and atom of copper - 29Cu:
1s22s22p63s23p64s13d10, instead of 29Cu: 1s22s22p63s23p64s23d9.
The number of electrons in a negatively charged ion - anion exceeds number of electrons in a neutral atom: 16S2 - 1s22s22p63s23p6 (18
electrons).
At formation of a positively charged ion - cation – electrons are
detouched first from the outer shell: 24Cr3 +: 1s22s22p63s23p64s03d3 (21
electrons).
Electrons in atom can be divided into two types: internal and
external (valence). Internal electrons occupy completely filled subshells,
have low values of energy and do not participate in chemical
transformations of elements.
Valence electrons are all electrons of the outer electronic shell as
far as electrons of not-filled subshells.
Valence electrons take part in formation of chemical bonds.
Unpaired electrons have special activity. The number of unpaired electrons
defines the valence state of a chemical element.
In case if not filled orbitals are available on the highest energetic
level, unpairing of valence electrons may occur, and the valence state of the
atom increases (formation of an excited state of the atom takes place).
For example, valence electrons of sulfur are 3s23p4:
16S
3s
3p
3d
In the ground state, the S atom has 2 unpaired electrons, so its
valence state is II.
At an expense of some energy one of paired electrons of sulfur can
be translated on an empty d-orbital, that corresponds to the first excited
state of the atom:
16S*
3s
3p
3d
In this case, the S atom has four unpaired electrons, and its valence
state equals IV.
One of 3s- paired electrons can also be moved to a free 3d-orbital:
16S **
27
3s
3p
3d
In such a condition, the S atom of sulphur possesses the valence
state VI.
If the outer electronic shell has no free orbitals or subshells,
unpairing of electrons is not possible and the atom can have onlt one
valence state (example – O-atom):
8О
2s
2p
THE PERIODIC LAW AND PERIODIC TABLE OF
CHEMICAL ELEMENTS
In 1869, the Russian chemist Dmitry Mendeleyev has shown that
properties of simple substances, and forms and properties of chemical
compounds of elements are in periodic dependence on nuclear scales of
elements. As expression of this periodic law, the table, which reflects the
law, was served.
In 1914 the English scientist G.Mozli has shown, that the charge of
a nucleus of an atom is numerically equal to an element serial number in
the periodic table, so properties of elements and their compounds are in
periodic dependence on a nucleus charge of the atom.
The periodic table of elements reflects electronic structures of
atoms. Each period (a horizontal series of periodic table) begins by an
element in which electrons start occupying a new electronic shell with the
principle quantum number that equals the number of the period).
Groups (vertical columns) contain elements with identical number
of valence electrons that equals the group number. Groups A contain selements (valence electrons occupy s-subshells). In case if valence
electrons are on s - and p-subshells, they carry the name of p-elements.
Elements with not fulfilled d - or f-subshells, are known as d - and felements. They occupy groups B of the periodic table.
28
Change of properties of chemical elements in the periods and
groups of the Periodic table
Chemical properties of elements are illustrated by interactions of
their atoms.
Properties of chemical elements can be divided into metallic
(reducing, i.e. properties to lose electrons) and nonmetallic (oxidising, i.e.
properties to gain electrons).
Properties of chemical elements depend on strengths of attraction
of valence electrons to a positively charged nucleui of atoms and are
defined by following characteristics:
Ionization energy (Ei) is an energy that is necessary for spending
for a separation and removal an electron from atom, an ion or a molecule.
Ionization energy is a measure of metallic (reducing) properties of
elements: the lower the Ei, the stronger the metallic properties are. In
groups at increase in a serial number of an element, the ijnization energy
decreases, and in period - increases.
Energy of electron affinity (Ea) is an energy that is allocated at
joining an electron to an atom or a molecule. It characterises non-metallic
(oxidising) properties of elements: the greater the value Ea, the stronger the
non metallic properties are. In the periods from left to right energy of
electron affinity and non-metallic (oxidising) properties of elements
increase, and in groups from up to down they decrease.
The half-sum of ionisation energy and energy of electron affinity is
called electronegativity of atom. It increases with increase in non-metallic
properties of elements.
In the periodic table, non-metallic elements are settled down in
groups A and occupy its right top part. Metallic elements of groups A are in
the left bottom part of periodic table. All elements of groups B possess
metallic properties.
29
CHEMICAL BONDS
The structure of chemical compounds is defined by the nature of
chemical bonds.
The chemical bond arises at the interaction of atoms causing
formation of chemically steady two-or multinuclear system (molecule,
crystal, etc.). The formation of a chemical bond is connected with the
general decrease in energy of a system of co-operating particles.
The major characteristics of a chemical bond are bond energy,
bond length, bond angles.
Bond energy is a quantity of energy allocated at formation of a
chemical bond. The more the bond energy, the stronger the molecule is.
Bond length is a distance between nuclei of atoms in a molecule.
Bond lengths are caused by sizes of reacting atoms and degree of overlap
of their electronic shells.
On a way of formation, three principal types of a chemical bond
are distinguished. These are ionic, co-valent, and metallic.
Covalent bond
The chemical bond between the atoms carried out by shared
electron pairs is called a covalent bond. It arises between identical atoms
forming gaseous binuclear molecules, and also in the condensed state with
participation of non-metallic atoms.
There are two basic concepts of description of a co-valent bond:
1. Method of valence bond (VВ).
2. Method ща molecular orbitals (МО).
30
Both methods mutually supplement and do not exclude each other
as they use various ways of approaches.
Method of valence bonds
According to the VB method, valency can be considered as
number of formed shared electronic pairs. From the point of view of the
exchange mechanism, valency of an element is defined by number of nonpaired electrons.
Atoms can form a limited number of chemical bonds according to
their valency. It corresponds to saturability of a covalent bond.
Depending on number of unpaired electrons, atoms can form one,
two or three co-valent bonds, i.e. a co-valent bond may be simple, double
or triple.
The strongest chemical bonds arise in a direction of a maximal
overlap of atomic orbitals. As the orbitals have different spatial
dispositions, therefore co-valent bonds are characterised by orientations.
Depending on directions of overlapping, one can distinguish ,
and -bonds.
-bonds are formed when two atomic orbitals overlap along an
axis connecting nuclei of atoms.
-bonds are formed when two atomic orbitals are site-overlapped.
-bonds arise at overlapping of two d-orbitals, located in parallele
planes.
The hybridization process also influences orientation of a covalent bond.
Hybridization is a mixing of of different subshells of an atom,
electrons of which participate in formation of equivalent chemical bonds.
Depending on hybridization type, hybrid orbitals have different
position in space.
31
sp - linear, an interbond angle 180
sp2 - triangular, an interbond angle 120
sp3 – tetrahedral, an interbond angle 109
Shared electron pairs in a molecule are shifted to a more
electronegative atom, thus a co-valent bond possesses a property of
polarity. Molecules formed by identical atoms (Cl2, H2, etc.), have nonpolar bonds. The more the difference of electronegativities of the two
atoms forming a chemical bond, the more polar it is.
In case if an exchange mechanism of formation of a co-valent
bond takes place, one of atoms (donor) delivers a pair of electrons, and
another (acceptor) – a not-filled orbital. Therefore, a co-ordinate bond is
formed.
For example, formation of an ammonium ion (NH4 +) involves
realization of both mechanisms of formation of a co-valent bond.
The
electronic
formula
of
a
nitrogen
atom
is
2s
2p It has five electrons on the valence shell
2 2
3 1s
7N 1s 2s 2p .
of which three electrons are unpaired and form covalent bonds with three
H-atoms (exchange mechanism). The lone electron pair of nitrogen
participates in formation of a co-ordinate bond with an ion Н+ (nitrogen
represents itself as the donor, and ion Н + as an acceptor of electrons).
The method of valence bonds allows distinguishing concepts of
valency and oxidation state.
Valency of an atom characterises its ability to form co-valent
chemical bonds.
Oxidation state is a conditional charge on atom in a molecule if to
assume, that shared electronic pairs are completely shifted to a more
electronegative atom.
32
For example, valency of nitrogen in molecule NH3 is III (three covalent bonds). Since electronegativity of nitrogen exceeds that of hydrogen,
all three formed shared electronic pairs are shifted towards nitrogen, giving
to it a negative charge (oxidation state –3).
The VB method ВС theoretically predicts structures and properties
of many molecules and ions. Therefore, it cannot explain existence of some
molecular ions (He2+, O22- ets). By means of this method, it is impossible to
explain magnetic properties of some molecules, for example, О2 and В2.
Method of molecular orbitals (МО)
According to МО method, the molecule consists of a set of nuclei
and electrons disposed on molecular orbitals.
Main tenets of the MO method:
1. Each electron in a molecule occupies a certain energetic level
(molecular orbital, MO) which is characterised by a molecular function and a corresponding set of quantum numbers.
2. The total number of formed МО equals the number of initial atomic
orbitals.
3. Filling of MO occurs according th all the principles presented for
atomic orbitals.
4. МО it is considered as a linear combination of atomic orbitals (MO –
LCAO).
Let us consider, for example, formation of molecule АВ. Valence
electrons of each atom are on p-orbitals.
If the wave function of the isolated atom A is А, and for the atom
B it is В, so according to the МО method:
АВ=С1АС2В
33
where С1 and С2 are coefficients considering the income of each atom in
formation of a molecular orbital.
.
А
E
В
(p*.)
_
А
В
+
А
В
(p)
The new molecular orbital with a lower energy (p) is known as a
binding orbital. As its energy is lower than the energy of an atomic orbital,
electrons on it stabilize the molecule.
The MO with a higher energy (p*) is called an antibinding
orbital. Electrons on it tend to destroy the molecule.
Stability of a molecule is described by a bond order, B.O.
B.O. = ½ (No of binding electrons – No of antibinding electrons)
If B.O. = 0 the number of electrons on binding orbitals equals the
number of electrons on antibinding electrons. Such molecule is unstable
and breaks up to initial atoms (does not exist).
Conditions for formation of MO from AO are the following:
close values of energies of overlapping atomic orbitals;
considerable overlap of AO (formation of and -types of MO);
identical spatial disposition of AO (рх - рх, instead of рх - рz).
34
On level power molecular орбитали two-nuclear molecules
settles down in a following order: s(1s) s(1s) s(2s) s*(2s)
x(2px) z(2pz) = y(2py) z*(2pz) = y*(2py) x*(2px)
Examples of the description of molecules using the MO method
(energetic diagrammes of molecules and molecular ions):
1. Molecule Н2
Energetic diagrammes shows transformation of atomic orbitals into
molecular orbitals.
e
e
e
Е
s*
*
1s
1s
s
Н-atom
Н2-molecule
Each H-atom has only
one s-orbital. While they
interact, two molecular
orbitals are formed.
According
to
the
principle of minimal
energy,
both
two
electrons in the H2
molecule occupy the sbinding
orbital.
1
The
s bond order is:
B.O. = ½(2 – 0) = 1
This correlates with the
single bond in H2
molecule from the point
of view of the VB
method.
Н-atom
35
2. Molecule Не2
As each He-atom has two electrons paired on 1s-atomic orbitals, in
the He2 molecule both binding and antibinding orbitals contain same
number of electrons. B.O. = 0, so the molecule does not exist.
Е
s*
*
1
s
1s
s
Не-atom
36
Не2-molecule
Не-atom
3. Molecular ion Не2+
One can suppose that a molecular ion Не2+ can be formed if a Неatom interacts with the Не+-ion. Totally, 3 electrons are present in the
spyce, two of which occupy a s-binding orbital and the rest one is
disposited on the s*-antibinding orbital. B.O.= ½, i.e. the molecular ion
Не2+ exists and forms a semi-bond from the point of view of the VBmethod. The existence of a Не+-ion was proved experimentally, aqnd it was
found that the bond between two nuclei is twice weaker than that in the H2molecule.
Е
s*
*
1
s
1s
s
Не-atom
Не2+molecular ion
Не+-ion
37
4 Molecule О2
Е
p*
p*
2p
p
p
s*
2s
2s
s
O-atom
38
O2-molecule
О-atom
Electronic configuration
of valence shell of Oatom is (2s22p4). The
interaction of two sorbitals of two oxygen
atoms is analogue to
previous cases.
p-Orbitals can form both
and bonds.
All 12 electrons of the
two O-atoms occupy
lower
molecular
orbitals. As two p*
molecular orbitals are of
same
energy,
O2 molecule
has
two
unpaired electrons and
possesses paramagnetic
properties.
Bond order B.O. = 2
which correlates with
the double bond from
the VB-method.
Ionic bonds
Ionic bond represents an electrostatic interaction between ions of
opposite charges. Ionic bond can be considered as a limiting case of a polar
co-valent bond where the difference of electronegativities of the two atoms
forming a chemical bond exceeds 2). Usually it is considered that a ionic
bond is formed at interaction of typical metals and typical non-metals.
Energy of ionic bonds depends upon:
1. energy of electrostatic interaction between ions, i.e. it increases with
increase in charges of ions and reduction of their radii;
2. energy of electronic affinity of non-metals which increases at increase in
non-metallic properties of elements;
3. ionisation energy of atoms.
Example: formation of a molecule of sodium chloride:
Na + Cl NaCl
Na Na + + e
Еi = 495 kJ
Cl + e Cl Еa = 345 kJ
Na + + Cl - NaCl Ecolomb = 585 kJ
Еbond = Еcolomb + Еa - Еi = 435 kJ
Ionic bonds are not directed and not saturable. That defines a great
stability of ionic crystals.
INTERACTION OF MOLECULES
(THE CONDENSED STATE OF SUBSTANCES)
Chemical stability of molecules is shown only in systems, where
distance between molecules is much more than their sizes (r10-9m). That
corresponds to a gaseous state of a substance.
39
In case if the distance between molecules makes about 10-9 m
(condencad state which may be liquid or solid) , arise forces of van der
Waals which have electrostatic nature and are subdivided on:
1) orientational (dipole - dipole);
2) inductional (dipole - not polar molecule);
3) polarising (dispersive interaction of instantly induced dipoles of
polarizable molecules).
Hydrogen bonds have intermediate character between intermolecular
interaction and a co-valent bond. It is a kind of interaction between
positively polarised atom of hydrogen and negatively polarised atoms with
high electronegativity (F, O, N, S, etc.).
At the expense of the small size of an H-atom, it has ability to enter
electronic shells of other atoms where there is an interaction that is
intermediate between electrostatic interaction and covalent bond
(interaction with lone electron pairs of non-metallic atoms).
Hydrogen bond is indicated as Х - Н … Y (X, Y =F, O, N, S).
CONCEPTS OF ADSORPTION
Adsorption - is the takeover of one substance onto another surface.
Absorption - is the takeover of one substance into another volume.
Noncompensative forces of attraction and repulsion of molecules of a
substance on the surface lead to the surface tension and the ability to adsorb
molecules from the environment:
40
By the type of interaction between the molecules of the surface
(adsorbent) and molecular environment (adsorbate), the adsorption can be
classified as following:
1. Physical adsorption (only associated with intermolecular
interactions. Such adsorption is reversible and is always accompanied by
desorption);
2. Chemical adsorption which is accompanied by the chemical
reactions on a surface, such as the occurrence of an oxide film on the metal
surface. This adsorption is irreversible.
The adsorption dependents on the temperature ( reduced by heating
) and the pressure ( increasing the pressure increases the adsorption of gas
phase).
The dependence of the adsorption of the adsorbate concentration
(or pressure) at constant temperature is called adsorption isotherm:
Г = f(P)T = Const или Г = f(С)T = Const
According to the theory of Langmuir, adsorption occurs only in the
places of maximum of attractive forces ( active sites ) . If all of the active
surface sites are occupied, the further adsorption does not take place. If we
assume that Г is the adsorption at any given time , and Г max is maximally
possible adsorption (all active sites of the adsorbent are employed by
adsorbate ) then
Г / Гmax = (surface coverage);
1 – - the degree of free surface capable of adsorption.
41
The adsorption rate is proportional to the concentration of the
adsorbate in the environment and the amount of free space on the surface of
the adsorbent ( proportion of empty seats ), and the desorption rate is
proportional to the number of seats occupied on the adsorbent surface :
v(ads.) = k(ads.)( 1 – ) [adsorbate]
v(des.) = k(des.) .
Upon reaching the equilibrium,
v(ads.) = v(des.);
k(ads.)( 1 – ) [adsorbate] = k(des.) ;
k(des.)/ k(ads.) = / ( 1 – ) [adsorbate] = К.
Then,
Г = Гmax K [adsorbate] / 1 + K [adsorbate]
At the initial moment of adsorption, the adsorbate concentration on
the adsorbent surface is small , ie 1 >> K [ adsorbate ] , the denominator is
close to unity , Г = Гmax K [ adsorbate ] , ie, the adsorption is linearly
dependent on the concentration of the adsorbate (line 1 in the graph) .
After all of the active adsorption sites are occupied, the
concentration of the adsorbate great 1 << K [ adsorbate ] , the denominator
can be taken for K [ adsorbate ] , that is, Г = Г max (adsorption is constant
and does not depend on the concentration of the adsorbate , which is
reflected line 2 parallel to the axis of the abscissa ) . In between, the
dependence of Г on the concentration of the adsorbate is nonlinear , which
is reflected on the adsorption isotherm :
Г
2
1
[adsorbate]
42
CONCEPT OF CATALYSIS
The process of changing the rate of a chemical reaction due to the
introduction of substances into the reaction system which is not part of the
reaction products is called catalysis. Positive and negative catalysis are
distinguished, ie the ones which accelerate or decelerate the rate of the
reaction. Catalysts are substances that increase the rate of reaction and
remain unchanged after it. Substances that slow down the rate of reaction
are called inhibitors.
Reactions in which one of the products is the catalyst of this
process , called autocatalytic .
The homogeneous and heterogeneous catalysis are distinguished.
In the case of homogeneous catalysis, the reactants and the catalyst are in
the same phase. An example is the oxidation of gaseous sulfur (IV) oxide
to a sulfur (VI) oxide with nitric (IV) oxide as a catalyst. The phenomenon
of homogeneous catalysis may be explained by the theory of intermediate
compounds , according to which in the presence of a catalyst the reaction
proceeds in several stages. Schematically, this may be expressed as :
A + B = AB (reaction without catalyst is slow ) .
In the presence of a catalyst :
1-st Stage
A + K = AK ( fastly. AK - Intermediate )
2nd Stage
AA + B = AB + K ( Catalyst K after the reaction
remains chemically unchanged ) .
The main reason for accelerating action of homogeneous catalysts
is to reduce the activation energy necessary for the reaction.
In heterogeneous catalysis, the reactants and catalyst are in
different phases (generally , the catalyst is a solid , the surface of which
participates in the acceleration of the reaction) . An example is the reaction
of oxidation of sulfur oxide (IV) with oxygen on the platinum surface .
Heterogeneous catalysis is due to the adsorption of the reactants on the
catalyst surface ( active areas ) . Increasing of the surface concentration
increases the reaction rate. The reaction products are desorbed from the
catalyst surface .
43
The rate of heterogeneous catalysis as the adsorption rate will be
determined by the number of active sites on the catalyst surface , and a
graph similar to the adsorption isotherm .
The heterogeneous catalyst is always more active than the
homogeneous catalyst (largely increases the reaction rate) .
Biological ( enzymatic ) catalysis - a catalysis of biochemical
reactions using biocatalysts - enzymes.
Features of enzymatic catalysis:
1. The high catalytic activity of enzymes ( in hundreds times more
active than the inorganic catalysts) .
2. Biocatalysts unlike inorganic catalysts have high specificity ( a
single enzyme is generally catalyzes a biochemical reaction).
3. The need to create special conditions (even a small change in pH
and temperature leads to a change in the catalytic properties of enzymes) .
44
SOLUTIONS
Solutions are homogeneous systems of variable composition.
Solutions consist at least of two components - solvent and solute.
Solvents are accepted to be that substances which keep their aggregate
states or which are of greater amounts.
Amount (mass) of the solute in a mass or volume unit of the
solution is nown as concentration of a solution.
The most widespread concentration units of solutions are rhe
following:
Mass fraction represents a mass of substance in 100 g of a
solution:
m( solute)
m( solution)
(100%)
Molar concentration (molarity) is a number of moles of a solute
in one liter of a solution:
CM
( solute)
V( solution)
m( solute)
M ( solute) V( solution)
Equivalent (normal) concentration is a number of equivalents of a
solute in one liter of a solution:
CE
n( solute)
V( solution)
m( solute)
M E ( solute) V( solution)
Solubility is an ability of one substance to be dissolved in other
under the set conditions. Quantitatively it is expressed by solubility factor,
s. It equals concentration of the saturated solution under the given
conditions.
Solubility of substances depends on temperature and pressure: for
liquid and solid solutes, it increases at rise in temperature, for gases - at fall
of temperature and pressure increase.
45
Physical and chemical processes in solutions
Interaction between molecules and ions of molecules of solute and
solvent can consist of the several processes proceeding consistently or
simultaneously.
1. Molecular dissociation of a solute:
(АВ)k
k AB
2. Interaction of molecules of solute with molecules of solvent
(formation of solvates):
AB + (n+m) S
AB (n+m) S
3. Electrolytic dissociation (splitting of a solute into solvated
ions):
AB (n+m) S
Ax + nS + Bx mS
Substances which can form ions while being dissolved, are known
as electrolytes.
The quantitative characteristic of electrolytic dissociation is known
as degree of dissociation:
= Сdis / Сtot,
where Сdis is the consentration of dissociated part of the electrolyte, and
Сtot is its total concentration.
According to the value of the degree of dissociation, the
electrolytes can be devided into two groups:
1. Strong electrolytes (> 0.3 or 30 %). Among strong electrolytes
there are some strong acids (HCl, H2SO4, HNO3, HClO4, HBr, HI), alkalis
(soluble bases such as NaOH, KOH, Ca(OH)2, Ba(OH)2, etc.) and
practically all salts. In solutions, strong electrolytes are practically
completely broken up to ions (dissociation is irreversible and complete):
2
Al2 (SO4) 2 Al3 + + 3 SO4
46
2. Weak electrolytes ( 0.03 or 3 %). Among weak soluble
electrolytes there are weak acids, ammonium hydroxyde NH4OH, and
water itself. Dissociation of weak electrolytes is a reversible and stepwise
process that is characterised by stepwise and overall equilibria constants
(dissociation constants).
For example, dissociation of phosphoric acid is a three-step
process:
[H ] [H2 PO4 ]
H + + H2PO4 ;
1-st step: H3PO4
2-nd step: H2PO4
[H3 PO4 ]
K1 =
[H ] [H PO4
H + + HPO4
2
2
H + + PO4
3
[H2 PO4 ]
K2 =
2
;
2
;
[H ] [ PO4
3-rd step: HPO4
=8103
K3 =
3
[ HPO4 ]
]
=6108
]
=21012
Overall process:
[ H ]3 [ PO4
H3PO4
3H + + PO4
3
;
K=
2
[H3 PO4 ]
3
3
]
= К1К2К3=11021
where [H+], [H2PO4 ], [HPO4
], [PO4 ], [H3PO4] are equilibrium
concentrations of ions; К1, К2, К3 – stepwise dissociation constants; and
K is the overall dissociarion constant.
47
Reactions of ionic exchange
Reactions of ionic exchange in solutions occur between ions of
strong electrolytes and molecules of weak electrolytes and insoluble
substances. They proceed towards formation of precipitates, gases, and
molecules of weak electrolytes.
Na2SO4 + 2HNO2
2NaNO2 + H2SO4
soluble
strong
soluble
weak
(reaction in a molecular form)
2Na + + 2NO2
2Na + + SO42
+ 2H + + SO42
(full ionic form of the reaction)
2NO2
+ 2HNO2
2HNO2
+ 2H +
(net ionic form)
The character of the dissociation defines the properties of chemical
compounds in solutions:
HCl H + + Cl (acids form hydrogen ions Н + while dissociation);
NaOH Na + + OH
NaCl Na + + Cl
(bases dissociate to produce ions of hydroxide OH );
(salts form metallic cations and anions of acids).
The main reaction that reflects acidic and basic properties is the
reaction of neutralization (acids interact with bases to produce salts):
Na2SO4 + 2H2O
2NaOH + H2SO4
2Na + + 2OH
48
2Na + + SO42 + 2H2O
2H2O
2OH + 2H +
+ 2H + + SO42
There are electrolits that can participate in chemical reactions both
as bases and as acids. Such electrolytes are called amphoteric. Among
them there are Zn(OH)2, Pb(OH)2, Sn(OH)2, Be(OH)2, Al(OH)3, Cr(OH)3,
As(OH)3, and some others. These substances are capable to react both with
acids and with the bases, forming salts as products of reaction of:
Al(OH)3 + 3HCl AlCl3 + 3H2O
Al(OH)3 + 3H+ Al3+ + 3H2O
Al(OH)3 + 3NaOH Na3 [Al(OH)6]
Al(OH)3 + 3OH- [Al(OH)6]3Sn(OH)2 + 2HCl SnCl2 + 2H2O
Sn(OH)2 + 2H+ Sn2+ + 2H2O
Sn(OH)2 + 2NaOH Na2 [Sn(OH)4]
Sn(OH)2 + 2OH- [Sn(OH)4]2-
DISSOCIATION OF STRONG ELECTROLYTES
In solutions of strong electrolits owing to their full dissociation,
concentration of ions is great; therefore, properties of such solutions will
depend on degree of interaction of ions as with each other, and with polar
molecules of solvent. So, concentreations of ions are replaced by their
activities.
Activity is a visual concentration of an ion involving its interaction
with other ions of the solution:
a = fC (f - activity factor).
If f = 1 ions are free and do not co-operate among themselves
(a=C).
If f <1 ions co-operate (a <C). The less the activity factor, the more
interaction between ions exists in the solution.
49
The activity factor depends on total concentration of all the ions in
a solution (ionic strength of a solution):
= l Ci Zi2,
where - ionic strength; Ci - concentration of ions in a solution; Zi charges of ions.
log f 0.5Z 2
1
(Debaue-Huckel’s equation)
For diluted solutions of strong electrolytes with <<1,
log f 0.5Z 2
DISSOCIATION OF WEAK ELECTROLYTES
While dissociation of weak electrolytes takes place, equilibrium is
established.
CH3COOH
CH3COO + H +
Thus, if the total concentration of the electrolyte equals C, and
degree of its dissociation is ,
Cdis = ∙ C
[CH3COO-] = [H +] = C
[CH3COO-] = C – C
K
H CH COO
3
CH 3COOH
For 1 K=C2
50
C 2 2
C 2 2
C 2
C C C 1 1
and
K
C
The resulted equation expresses the Ostwald’s dilution law
according to which degree of dissociation of a weak electrolyte increases
with dilution of a solution.
Addition of common ions in a solution of a weak electrolyte causes
shifting of equilibrium of the reaction towards reduction of dissociation
(effect of a common ion).
ELECTROLYTIC DISSOCIATION OF WATER
Water is a weak electrolyte that dissociates according to the
equation:
Н2О
Н + + OH.
o
At the temperature of 22 C, the equilibrium of the dissociation
process establishes such that:
[H +] [OH ] = KH2O = 1014
(ionic product of water).
In a neutral solution [H +] = [OH-] =
In an acidic solution [Н +]> [OH-];
In an alkaline solution [H +] <[OH-];
1014
= 107 mol/l.
[H +]> 107 mol/l.
[H +] <107 mol/l.
Knowing concentration of one of the ions, for example [Н +] and
ionic product of water, it is possible to calculate concentration of another
type of ions [OH-].
The negative logarithm of concentration of of hydrogen ions (or the
negative logarithm of activity of ions of hydrogen) is named рН:
рН = – log [H +]
In neutral solutions at 22оС рН = 7.
In acidic solutions рН < 7.
In alkaline solutions рН > 7.
51
Acid-base indicators are substances, changing colouring in certain
area of value pH a solution. Weak organic acids or bases, which molecules
and ions have different colouring can be indicators.
Area of transition of colouring of some indicators
The indicator
Methylorange
Phenolphtalein
Litmus
Colour
Area of
transition of
colouring, рН
acidic form
red
colourless.
red
alkaline form
yellow
red
dark blue
3.2 – 4.5
8.2 – 10.0
6.0– 9.,0
EQUILIBRIA IN SOLUTIONS WITH PRECIPITATES
In the saturated solution of a sparingly soluble electrolyte, dynamic
equilibrium between a firm phase and a solution is established which can be
presented by the equation:
CaCO3 (s)
Ca2 + + CO32
For this equation, using the law of mass action, we can write down
the expression of equilibrium constant:
[Ca 2 ][CO3 2 ]
[CaCO3 ]
Ks =
where [Ca2 +], [CO32 ] are equilibrium concentrations of ions in the
solution;
[CaCO3] is the concentration of the electrolyte in a precipitate (in a
firm phase), it is constant.
52
Having increased Ks on a constant [CaCO3], we will receive a
constant named solubility product, Ksp:.
Ks [CaCO3] = Ksp = [Ca2 +] [CO32 ]
Solubility product is a product of concentration of ions of a sparingly
soluble electrolyte in its saturated solution in their degrees of stoichiometric
factors. Numerical values of the solubility product of sparingly solyble
electrolytes are presented in special help tables.
In the presence of the common ions, the eqiolibrium is displaced
towards deposit formation (effect of the common ion). In the presence of
the strong electrolytes with no common ions, the mobility of ions in the
solution decreases, and the equilibrium is shifted towards an increase in the
precipitate dissolution (salt effect).
At mixing of the solutions containing ions giving an insoluble salt,
the precipitation starts. During the first moments of time, the concentrations
of ions in a solution are high, and the precipitation takes place. Within time,
gradually concentrations of ions decrease and an equilibrium is established.
Condition for formation of a precipitate:
the product of
concentration of ions in the solution exceeds the value of solubility product
of the given compound. For example,
[Ca2 +] [CO32 ]> Ksp (CaCO3).
Condition for dissolution of a precipitate: the product of
concentrations of ions in the solution deceeds the value of the solubility
product of the given compound ([Ca2 +] [CO32 ] <Ksp (CaCO3)).
53
Dependence of the solubility product and solubility
of sparingly soluble electrolytes
Consider the dissolution scheme of a sparingly soluble electrolyte of
the KxAy type as
KxAy
x Ky + + y Ax
The expression of the solubility product for this equation looks like:
Ksp = [Ky +] x [Ax ] y
Let us designate the molar solubility of the electrolyte as “S”. Then
the solution will contain cations in the concentration [Ky +] = xS (mol/l),
ans anions in the concentration [Ax ] = yS (mol/l). If to substitute these
designations in expression of the solubility product:
Ksp = [xS] x [yS] y,
From here, we can find the solubility S:
s x y
K sp
xxy y
BUFFER SOLUTIONS
Buffers are used to maintain a constant pH value in the sample
solution upon addition thereto of small amounts of strong acids, strong
bases, or dilution.
As the buffer solutions, a mixture solution of weak acids or weak
bases and their salts or mixtures of salts of polybasic acids with different
degrees of substitution usually used. The following table shows examples
of the most commonly used buffers and pH, they support:
54
Composition of the buffer solution
A mixture of CH3COOH
andСН3СООNa
The mixture NаН2РО4 and
Nа2НРО4
A mixture of NH4OH and NН4С1
The name of the
buffer
acetate buffer
рН
phosphate buffer
6.5
ammonia buffer
9.25
4.7
Buffer systems can bind both H + and ОН ions at the addition of
strong acids and bases to produce weak electrolytes that slightly alterin the
pH of the solution.
Example: Acetate buffer solution comprises a mixture of
CH3COOH and CH3COONa. The dissociation of a weak electrolyte - acetic
acid – is reflected by the reaction equation:
CH3COOH
CH3COO + H+
and is described by the equilibrium constant:
K a=
[CH 3 COO ] [ H ]
= 1.8 10 5
[CH3 COOH ]
By adding sodium acetate, the concentration of CH3COO ions
increases and is determined by the concentration of the salt:
[CH3COO ] Csalt.
The dissociation of a weak electrolyte is reduced by the
introduction of a common ion, so
[CH3COOH] Cacid.
Thus,
C
K a=
[H ]
salt
;
C
acid
55
[H+] = Ka
C
acid ;
C
salt
C
pH = –log[H+] = pKa – log ( acid ),
C
salt
where pKa = - log Ka.
Thus, pH of a a buffer solution is not dependent on the
concentrations of the components but is determined by their mole ratio.
By adding small amounts of a strong acid or a strong base (alkali),
the components of the buffer solution translating them into weak
electrolytes:
Examples.
1. At addition of NaOH, it can react with the qacetic acid:
CH3COOH + NaOH = CH3COONa + H2O
(the salt concentration increases accordantly to the concentration of the
added amount of the alkali, and the concentration of the weak acid
decreases to the same value):
pH = –log[H+] = pKa - log (
С
acid
NaOH ).
C
C
salt
NaOH
C
2. The addition of a strong acid provokes its interaction with the
sodium acetate:
CH3COONa + HCl = CH3COOH + NaCl,
C
pH = –log[H+] = pKa - log ( acid
С
NaOH ).
C
C
salt
NaOH
Since the change of the ratio of the concentrations is much less than
their sum or the difference, the total the pH value changes insignificantly.
56
The amount of a strong acid or a strong base to be added to the
buffer solution to change the pH of one liter of a solution thereof to a unit is
called a buffering capacity (B). It can be calculated with respect to the acid
(Ba) or base (Bb).
C a Va
( pH1 pH 2 ) V
solution
C V
b b
Bb=
( pH 2 pH1 ) V
solution
Ba=
wherein Ba and Bb - buffering capacity for the acid and base
respectivelyy; Ca and Cb - concentration of added acid and base; pH1
and pH2 - initial and final pH of the solution; Va and Vb – volumes
of the added strong acids and bases.
DIRECTION OF REACTIONS OF IONIC
EXCHANGE
Reactions of ionic exchange are irreversible only in that case when
weak electrolyites or sparingly solyble electrolytes exist among the
products. In that case, when weak electrolytes and precipitates are present
both among the reactants, and among the products, the reactions of ionic
exchange are reversible and are shifted either towards the products (the
reaction is possible to proceed) or towards the reactants (the proceeding of
the reaction is not possible). For determination of the possibility of
proceedings of such reactions, the equilibrium constant must be calculated.
Rule for calculation of the equilibrium constant. To calculate an
equilibrium constant of the reaction, write down the product of all the
57
possible constants for the compounds among the products of the reaction in
the numerator of the fraction expressing equilibrium constant (by the
possible constants, the solubility products of sparingly soluble electrolytes,
dissociation constants of weak electrolytes - acids or the bases, instability
constants of of complex ions, ionic product of water are supposed), as well
as the same for reaction products should be added to the fraction
denominator, ranking all the constants in the degrees corresponding to the
stoichiometric factors of the corresponding substance in the equation of the
reaction. The multibasic acids are represented by their overall dissociation
constants which are products of their stepwise constants.
In the case if the equilibrium constant of the reaction,
К(reaction)> 1,
the equilibrium of the reaction is shifted towards the products, and the
reaction of ionic exchange is possible.
In the case if
К(reaction)<105,
the equilibrium of the reaction is practically completely shifted towards the
reactants, and the reaction proceeding is impossible.
In tha case if the К(reaction) is concluded in limits from 1 to 105,
the reaction proceeding becomes possible in excess of one of the reagents.
Example. Let us determine the possibility of dissolution of CaCO3
in the acetic acid.
The molecular reaction of the process can be written as:
СaCO3 + 2 CH3COOH
Ca (CH3COO) 2 + H2CO3
In the net ionic form the reaction is presented as following:
СaCO3 + 2 CH3COOH
58
Ca2 + + 2 CH3COO - + H2CO3
The equilibrium constant of the reaction may be calculated according to
the formula and using the corresponding table data:
Ksp(CaCO3 ) K 2 (CH 3COOH )
K (reaction)
6.6 10 2 .
1
2
K ( H 2 CO3 ) K ( H 2 CO3 )
The value of the К(reaction) specifies that calcium carbonate can
be dissolved in excess of acetic acid.
HYDROLYSIS OF SALTS
If the salt dissolved in water contains ions-rests of weak acids or
the weak bases there is a process of hydrolysis of salt - exchange reaction
of ions of salt to molecules of the water leading to formation of molecules
and ions of new weak electrolits.
Key rules for writing the reactions of hydrolysis:
1. Only anions of weak acids and cations of weak bases which are a
part of the salt are exposed to hydrolysis process.
2. Hydrolysis is a stepwise process. At each step, one hydrolyzed
ion reacts with one molecule of water.
3. Under common conditions, hydrolysis proceeds only on the first
step. Hydrolysis amplifies at heating and dilution of the solutions of the
salts.
4. As a rule, hydrolysis is a reversible process. Its equilibrium it is
possible to be displaced by addition of small amounts of strong acids and
alkalis.
59
Types of hydrolysis reactions.
1. Salt is formed by the ions of a strong base and a strong acid
(for example, NaCl, KNO3, etc.).
NaCl + H2O hydrolysis does not proceed (NaOH is a strong
base, HCl is a strong acid).
2. Salt is formed by the ions of a strong base and a weak acid (for
example, Na2CO3, KSCN, etc.).
Na2CO3 + Н2О anions are involved in hydrolysis (NaOH is a
strong base, H2CO3 is a weak acid).
CO32 + HOH
Na2CO3 + HOH
HCO3 + OH (alkaline medium, рН> 7).
NaHCO3 + NaOH (1 step of hydrolysis).
Addition of alkalis (NaOH) to the above solution (OH are formed
within hydrolysis), causes depression in hydrolysis. Countrary, addition of
acids strengthens hydrolysis at the expense of reaction Н ++ OH Н2О
in which result concentration of ions OH in a solution decreases, and
balance of hydrolysis is shifted towards the products. Hydrolysis amplifies
and the second step becomes possible:
HCO3 + HOH
NaНCO3 + HOH
H2CO3 + OH
H2CO3 + NaOH (2-nd step of hydrolysis).
3. Salt is formed by the ions of a weak base and a strong acid (for
example, AlCl3, FeSO4, etc.).
AlCl3 + H2O cations are involved in hydrolysis (Al(OH)3 is a
weak base, НCl is a strong acid).
Al3 + + HOH
AlOH2 + + H + (alkaline medium, рН <7)
AlCl3 + HOH
AlOHCl2 + HCl (1-st step of hydrolysis).
4. Salt is formed by the ions of a weak base and aweak acid:
The salt is water soluble (for example, (NH4)2CO3, NH4NO2, etc.).
60
(NH4)2CO3 + H2O both cations and anions are involved in the
hydrolysis process:
2NH4 + + CO32 + HOH
NH4OH + HCO3 + NH4 + (рН 7)
(NH4) 2CO3 + HOH
NH4OH + NH4HCO3
The salt is insoluble in water (for example, FeS, ZnSiO3, etc.).
FeS + H2O insoluble salts do not undergo hydrolysis.
Some salts are irreversibly destroyed by water (for example, Al2S3;
Al2(CO3)3; Cr2S3; Cr2(CO3)3; Fe2S3; Fe2(CO3)3):
Fe2S3+6H2O 2Fe (OH) 3 +3H2S
Calculations of pH values of the solutions of the salts
Quantitatively hydrolysis is described by hydrolysis constant (Kh)
and degree of hydrolysis (h).
NaCN + H2O
NaOH + HCN
CN - + H2O
OH - + HCN; pH> 7
It is possible to express a hydrolysis constant through concentration
of ions in a solution taking into account that concentration of water
practically does not change, or by a rule of calculation of an equilibrium
constant of a reversible reaction of hydrolysis:
Kh=
KH O
[OH ][ HCN ]
2
K
[CN ]
acid
NH4Cl + H2O
NH4OH + HCl
NH4 + + H2O
H + + NH4OH; pH <7
61
[ H ][ NH 4OH ] K H 2O
Kh =
K base
[ NH 4 ]
NH4CH3COO + H2O
NH4 + + CH3COO
Kh =
NH4OH + CH3COOH
+ H2O
NH4OH + CH3COOH
KH O
[ NH 4OH ][CH 3COOH ]
2
K
[ NH 4 ][CH 3COO]
acid K base
Degree of hydrolysis “h” defines that part of salt, which has
undergone to hydrolysis under the given conditions:
h
С
hydrolyzed
.
С
total.
Hydrolysis degree is related to a hydrolysis constant:
Kh =
h 2C
.
1 h
In the case when h <<1, Kh = h2C.
Example. Calculation of the рН of a 0.1 M solution of potassium
phosphate.
Let us consider, that the hydrolysis basically proceeds on the first
step:
PO43
Kh =
62
+ HOH
HPO42 + OH
KH O
10 14
2
=
= 7.69x10-3
12
III
1,310
K H PO
3 4
h=
Kh
C K PO
3 4
=
7.6910 3
=
0 .1
7.69 10 2 = 2.77 10-1
[OH ] = hC = 2.77 10-1 * 0.1 = 2.77x 10-2;
[H +] = 10-14 / [OH ] = 10-14 / 2.77 10-2 = 3.61 10-13;
рН = -log [H +] = 12.44.
Irreversible hydrolysis
The equilibrium of hydrolysis reactions in some cases may be
irreversibly shifted towards the products iif a product of one of hydrolysis
steps will be sparingly soluble:
Bi (NO3) 3 + HOH
BiOH (NO3) 2 + HNO3
Bi3 + + HOH
BiOH2 + + H +
At dilution of the solution, the equilibrium of the reaction is shifted
to the right at the expense of formation of a precipitate of bithmuth oxonitrate:
BiOH(NO3) 2 + HOH Bi(OH) 2NO3 + HNO3
BiONO3 + H2O
BiOH2 + + HOH + NO3
BiONO3 + H +
The hydrolysis of antimony(III) chloride proceeds similarly.
63
Mutual hydrolysis
Ions Н + (or OH ) can combine together into water molecules in
the case when two salts with different types of hydrolysis are mixed
together. That will cause mutual strengthening of hydrolysis of both salts
and as a result - formation of products of hydrolysis (mutual hydrolysis).
For example, at mixing of solutions Na2CO3 and AlCl3 in which
accordingly there is a surplus of i OH and Н + ons accordingly, mutual
strengthening of hydrolysis leads to allocation СО2 and formation of a
precipitate of Al(OH)3:
2AlCl3 + 3Na2CO3 + 3H2O 2Al(OH)3 + 3CO2 + 6NaCl
2Al3 + + 3CO32
+ 3H2O 2Al(OH) 3 + 3CO2
In other similar cases, a least soluble of possible products of
hydrolysis is formed while a mutual hydrolysis. For example, the solubility
of a copper(II) hydroxo carbonate (CuOH)2CO3 is less than the one of
copper hydroxide Cu (OH)2. Therefore, mixing of solutions of CuSO4 and
Na2CO3 will lead to formation of (CuOH)2CO3:
2CuSO4 + 2Na2CO3 + H2O (CuOH)2CO3 + CO2 + 2Na2SO4
2Cu2 + + 2CO32 + H2O (CuOH)2CO3 + CO2
Mutual hydrolysis is an irreversible process.
COLLOIDAL SOLUTIONS
Colloidal solutions are ultra-microheterogenous systems
consisting of two phases: a dispersion medium and a dispersed phase. The
dispersed phase comprises colloidal solutions of molecular aggregates of a
size of 10-7 – 10-5 cm.
64
Classification of colloidal solutions
1. Physical state of the dispersed phase and the dispersion medium.
Dispersed
phase
gas
liquid
solid
Dispersion
medium
gas
liquid
solid
aerosols
aerosols
(clouds, fog) (smoke, dust)
emulsions
Sols,
(milk, oil) suspensions
(natural waters)
solid emulsion
gels
Litosols
(capillary system, (soil, pearls, (minerals,
activated carbon)
cheese)
alloys)
emulsions
(foam)
2. Interaction of the dispersed phase and the dispersion medium
(the type classification is used only for the liquid dispersion media) .
Colloidal systems are divided into lyophobic and lyophilic .
In the lyophobic colloidal systems, the dispersed phase weakly
interacts with the dispersion medium . Such systems can not be obtained
spontaneously dispersed phase always represent molecular aggregates
(irreversible colloids) .
In the lyophilic colloid systems (reversible colloids), the dispersed
phase is soluble in the dispersion medium . Its particles are individual
molecules of large size (macromolecules).
65
Properties of lyophobic colloids
1. Heterogeneous system (the interface of the dispersed phase and
dispersion medium).
2. Colligative properties of the colloid practically do not differ
from those of the dispersion medium.
3. Cannot be divided by mechanical methods (sedimentation ,
filtration, etc.) .
4. Poorly pass through semipermeable membranes and films.
5. When light passes through colloid solutions, a Tyndall’s cone is
observed deu to diffraction of light on the colloidal particles.
6. Since the shortwave light diffraction occurs to a greater degree
than the diffraction wavelength radiation , the colorless colloidal solutions
are blue in reflected light, and red in the transmitted light.
7. The colloidal particles are charged and can move under the
influence of an electric field (electrophoresis).
Colloidal micelle structure and electrical properties of colloidal
systems
Colloidal particles are small in size and can be suspended
indefinitely. This determines the kinetic stability of colloidal systems . On
the other hand, a large colloidal particle surface defines excessive surface
tension and the tendency to "sticking" of colloidal particles, which reduces
the energetic stability of colloidal systems.
Stabilization of colloidal systems is due to the adsorption of the
dispersion medium or the electrolyte ions present in the solution. On the
border of the colloidal particles, the electric double layer is formed. Thus.
colloidal particles acquire the same charge , which prevents "sticking".
Ions that form the charge of colloidal particles are called potential
forming ions. The- counterions of the opposite charge are located in the
adsorption and diffusion layers of the micelle.
66
The ion exchange occurs between the adsorption and diffusion
layers. The colloidal particles are charged and the micelle electrically
neutral.
Example. The structure of the micelle of the colloidal solution of
silver iodide .
The formation of silver iodide can be represented as :
KI (excess) + AgNO3 AgI + KNO3
The micelle structure : {m[AgI] nI (n-x)K+} x xK+
where "m" is the number of molecules of silver iodide in the core; "N" is
the number of anions in the first adsorption layer of the colloidal particle ; n
+ x = m; typically m >> n.
If there is an excess of silver nitrate in the solution, the silver ions
are primarily grouped on the surface of the colloidal core constructing
additional crystal lattice of the solid phase and nitrate anions serve as
counterions.
On the surface of the micelle core an electric potential of the
interphase is generated ( - potential), which decreases with increasing
distance from the core surface by adsorption of counterions.
If placed colloidal particle in an electric field, the counterdiffusion layer is detached from the colloidal particles, and moved in the
direction of the corresponding electrode, and a colloidal particle - in the
opposite direction. The absorption and diffusion layers are devided by a
sliding surface). The potential on the sliding surface is called electrokinetic
potential or ( zeta ) potentials .
- potential is a measure of the stability of colloidal systems : if <
30 mV, coagulation (destruction) of the colloidal particles occurs.
Introduction of indifferent strong electrolytes into a colloidal
solution decreases the -potential which is related to the increase in the
concentration of counterions and contraction of the diffuse layer. In the
case where the concentration of the electrolyte is sufficiently high, the
isoelectric state ( = 0) can be achieved.
67
At introduction of a strong common electrolyte, there is an
additional adsorption of the potential forming ions on the surface of
colloidal particles , which leads to an increase of the - potential. After
reaching the maximum possible adsorption of a common electrolyte, the
latter starts to act as the indifferent electrolyte, ie the - capacity decreases
and the coagulation of colloidal particles occurs.
Coagulating ability of an electrolyte is characterized by the
coagulation threshold - the minimum concentration of the electrolyte ,
which causes coagulation of the colloidal solution. Coagulation threshold
decreases with increasing the charge of the coagulating ion (the ion charge
sign is the same that the charge of the counterions of the colloidal particle).
For the mono- , di-, and triply charged ions, the ratio of their coagulation
threshold is 1:11:72 .
In some cases, the coagulation process is reversible. The process of
reverse transformation from coagulate to colloidal solution is called
peptization or disaggregation.
The stability of lyophobic sols increased by adding small amounts
of solutions of high molecular compounds (e.g., gelatin solution, tannin et
al.). The protective action of solutions of high molecular compounds is due
to the formation of the protective layer which is adsorbed on the surface of
the colloidal particle. The characteristics of the protective action is
protective number - minimum amount ( mg ) of the solid substance that
prevents coagulation of 10 ml of the colloidal solution by adding a strong
electrolyte in an amount determined by the threshold of coagulation.
Properties lyophilic colloids (solutions of highmolecular
compounds , HMC)
1. Homogeneous systems.
2. They are formed spontaneously and are thermodynamically
stable .
3. Colligative properties are determined by the concentration of the
HMC.
68
4. The colloidal particles can be charged (ionic HMC) or be
uncharged.
5. Coagulation threshold of the electrolytes significantly exceeds
the one for lyophobic colloid systems . The mechanism of action is
associated with coalescers desolvation (salting) of HMC. In some cases, the
drops form a second liquid phase - structured liquid approaching the jelly
by properties. This phenomenon is called coacervation and is typical for
many proteins. The concentration of the HMC in coacervate drops
increases , but otherwise the solution - is reduced compared with the
original.
6. Concentrated solutions of HMCare characterized by high
viscosity.
Ways of preparation of colloidal solutions
1. The dispersion methods (grinding of large aggregates of
molecules up to the size of the colloidal particles):
a) mechanical dispersion (using mechanical devices - ball mills ,
grinders , etc.);
b) electrical dispersion (grinding with an electric current) is used
for the preparation of sols of metals and other conductive materials;
b) peptization method (the precipitate is washed with freshly
prepared stabilizer electrolyte solution . EXAMPLE . Peptization
of potassium hexacyanoferrate(II) by the oxalic acid solution:
K4[Fe(CN)6] + FeCl3 KFe[Fe(CN)6] + 3KCl
H2C2O4
2H+ + C2O42
{m[KFe[Fe(CN)6]] n C2O42 (n-x)H+} x xH+.
2. The condensation methods (formation of aggregates of
individual molecules, i.e. the formation of colloidal solutions in the
course of chemical reactions):
a) an ionic exchange reaction
3H2S (excess) + As2O3 As2S3 + 3H2O
69
{m[As2S3] nS2 (n-2x)H+} x 2xH+
b) redox reactions:
2HAuCl4 + 5K2CO3 2KAuO2 + 5CO2 + 8HCl + H2O
KAuO2 + HCHO Au + HCOOK + H2O
{m[Au] nAuO2 (n-x)K+} x xK+
2H2S + O2 S + 2H2O
2H2S + 7O2 2H2O + H2S5O6
{m[S] nS5O62 (n-2x)H+} x 2xH+
c) hydrolysis reactions:
FeCl3 + H2O
FeOHCl2 + HCl
FeOHCl2 + H2O
Fe(OH)2Cl + HCl
Fe(OH)2Cl FeOCl + H2O
Fe(OH)2Cl + H2O
Fe(OH)3 + HCl
{m[Fe(OH)3] nFeO+ (n-x)Cl}X+ xCl
{m[Fe(OH)3] nH+ (n-x)Cl}X+ xCl
OXIDATION-REDUCTION REACTIONS
(REDOX REACTIONS)
Oxidation-reduction are those which proceed with the transmission
of electrons from one particles (atoms, molecules or ions) to another
therefore the oxidation states of some elements in the given particles
change.
Oxidation state is a conditional charge of an atom in a molecule
provided that all polar bonds are considered as ionic.
70
Oxidation process is a process of gaining of electrons; it is
accompanied by increase in the oxidation state of an element.
Reduction process – is a process of gaining of electrons; it is
accompanied by reduction of the oxidation state of an element.
The oxidizer is an ion or a molecule which is taking away electons
from other elements of an ion or molecule. In the course of oxidationreduction reaction, the oxidizer reduces.
Reducer is an element, an ion or a molecule, providing electrons to
other elements, ions or molecules. In the course of oxidation-reduction
reaction, the reducer oxidises.
Typical oxidizers: active nonmetals (O2; F2; Cl2), concentrated
solutions of H2SO4; solutions of HNO3 of any concentration; KMnO4;
K2Cr2O7 and other substances containing elements in their highest
oxidation states.
Typical reducers: active metals; H2; C; CO; H2S; HI and other
substances containing elements in the minimal (negative) oxidation states.
Elements in intermediate oxidation state possess redox duality, i.e.
can show both properties of oxidizers, and properties of reducers (Na2SO3,
KNO2, etc.).
In oxidation-reduction reactions the following ions usually do not
change their oxidation staes: ions of alkaline and akline earh metals;
hydrogen ions (except reactions of metals with acids-non oxdizers); oxygen
ions (except reactions with participation of hydrogen peroxide, H2O2); the
ions forming molecules of acids or alkalis, defining reaction of the
environment.
In oxidation-reduction reactions, the following changes of
oxidation states of elements are usually observed:
Mn+2
7
K MnO4
4
Mn O2
(In the acidic environment, for example,
MnSO4, MnCl2, etc.)
(In the neutral environment)
71
6
K 2 MnO4
4
(In reactions with the metals of low activity, and
non-metals)
S0
(In reactions with the metals of average activity)
S O2
6
H 2 S O4
(In the alkaline environment)
concenhtrated
2
H2 S
(In reactions with active metals)
4
(Heavy metals and non-metals)
N O2
5
H N O3
concentrated .
1
N2 O
.
2
NO
(Active metals)
(Heavy metals and non-metals)
5
H N O3
diluted .
0
N2
.
(Active metals)
(Very diluted nitric acid with very active metals gives NH4NO3 as a product
of reduction (nitrogen in the oxidation state-3).
72
6
K 2 Cr2 O7
Cr 3
OH H
OH H
6
3
3
4
Na2 S O3
Mn 2
Mn 2
5
Na Bi O3
1
H2 O 2
1
H2 O 2
Cl
Sn+2
Pb+2
In the alkaline environment
(for example, Na3 [Cr (OH)6])
5
(In reactions with
K N O3
oxidizers)
6
(In reactions with
Na2 S O4 oxidizers)
7
(In the acidic
H MnO4
environment)
4
(In neutral and alkaline
Mn O2
environments)
(In the acidic
Bi 3
environment, for example,
Bi (NO3) 3)
2
(In reactions with
H2 O
reducers)
0
(In reactions with
O2
oxidizers)
0
(In reactions with strong
Cl 2
oxidizers)
Sn+4
Pb+4
[Cr ( OH )6 ]3
K 2 Cr O4
K N O2
In the acidic environment
(e.g., CrCl3)
Direction of oxidation-reduction reaction
Oxidation-reduction properties of substance define on their
oxidising ability, числено expressed through redoks - potential . The real
potential pays off on the Nernst’s equation:
0,059
= 0+ n log (Cox / C red),
73
Where 0 - standard electrode potential of the given process, n - quantity of
accepted electrons, Cox and C red - concentrations of the oxidised and
reduced forms accordingly.
The more value , the is stronger oxidising properties of the
oxidised form of the given compound.
The direction of the oxidation-reduction reaction is defined on the
electromotive force (E):
E = (ox) -(red)
If E> 0, the direct reaction is possible. If E <0, the direct reaction is
impossible (reaction goes to the opposite direction).
Standard electromotive force E0 is connected with standard
Gibbs’energy G
0
nFE
On the other hand, G
the reaction K:
0
0
= - G
0
defines on the equilibrium constant of
0
G = - 2.3RT log K
Thus,
nFE
0
= 2.3RT log K
0
At the temperature of 25 C (298 K), the equation becomes:
nE 0
log K = 0.059 .
Classification of redox reactions
All oxidation-reduction reactions can be divided into three groups:
1). Reactions of intermolecular oxidation-reduction are reactions
in which the exchange of the electrons occurs between the atoms of
different molecules: Fe + CuSO4 = FeSO4 + Cu.
74
2). Reactions of intramolecular oxidation-reduction- in such
reactions an oxidizer and a reducer are in the same substance:
2КClO3 = 2KCl + 3O2.
3). Reactions of disproportionation are those in which the same
atoms in molecules co-operate with each other as an oxidizer and a reducer
because these atoms have intermediate oxidation states:
3К2MnO4 + 2H2O = 2KMnO4 + MnO2 + 4KOH.
Composition of the equations of redox reactions
The method of half-reactions is based on drawing up of ionic
equations for processes of oxidation and reduction with the account of рН
environments of the given reaction. Strong electrolytes in the given method
are registered in the form of ions, and the weak electrolytes- in the form of
molecules. The ionic scheme includes ions and molecules showing
oxidation-reduction properties, and the ions characterising the environment
(Н + and water molecules in the acidic solutions, OH ions and water
molecules in the alkaline environment, and all of the above in the neutral
environment):
Ca + HNO3 (разб) NH4NO3 +...
Ca0 - 2 e Ca2 +
( 4)
NO3 + 10H + + 8 e NH4 + + 3H2O ( 1)
4Ca0 + NO3 + 10H + 4Ca2 + + NH4 + + 3H2O
4Ca + 10HNO3 (разб) 4Ca (NO3) 2 + NH4NO3 + 3H2O
Examples of some half-reactions with participation of the most
typical oxidizers and reducers:
Concentrated sulfuric acid
SO4
2
+
+ 8H + 6e = S + 4H2O
75
SO42
NO3
NO3
NO3
+ 4H + + 2e = H2SO3 + H2O
Nitric acid
+ 10H + + 8e = NH4 + + 3H2O
+ 4H + + 3e = NO + 2H2O
+ 3H + + 2e = HNO2 + H2O
NO3 + 2H + + e = NO2 + H2O
Manganese compounds
MnO4
MnO4
MnO4
Cr2O72
CrO42
+ 8H + + 5e = Mn2 + + 4H2O
+ 2 H2O + 3e = MnO2 + 4OH
+ e = MnO42
Chromium compounds
+ 14H + + 6e = 2Cr3 + + 7H2O
+ 4H2O + 3e = Cr (OH) 63 + 2OH
Hydrogen peroxide
H2O2 + 2e = 2OH
H2O2 + 2H + + 2e = 2H2O
2H + + O2 + 2e = H2O2
2H2O + O2 + 2e = H2O2 + 2OH
COMPLEX COMPOUNDS
Complex compounds are those which are formed of separate, rather
simple chemical particles capable to independent existence (cations, anions,
molecules). As examples of complex compounds we may mention
Na3[Co(NO2)6]; [Cu(NH3)4]SO4; K4[Fe(CN)6].
The elementary complex compound is the ammonium cation:
NH3 + H + = NH4 +
76
Structure of complex compounds
One of the atoms of the complex compound, usually positively
charged metal cation, occupies the central place in a complex (the central
ion or a complex forming ion). Neutral molecules or ions with an opposite
charge (ligands) form coordinate chemical bonds with the central ion. The
central ion and ligands form inner sphere of the complex compound (a
complex ion).
Oppositely charged ions are electrostatically connected with the
complex ion and neutralised its charge. They carry the name of outer
sphere of a complex. Outer and inner spheres of a complex are connected
by ionic bonds.
The total number of chemical bonds formed by the central ion with
the ligands, is called as coordination number of the central ion. The
coordination number depends on a complexing ion charge (for
monocharged ions it is usually 1, for dicharged - 4 or 6, for tricharged - 6
and above), and the size of the ion.
The number of the chemical bonds formed by one ligand, is is
known as its dentation. Mono, bi - and polydentate ligands are
distinguished. The dentation it is defined by the number of lone electron
pairs in the ligand molecule and their mutual disposition. For example, the
N atom in the ammonia molecule has one lone electron pair, therefore
ammonia is a monodentate ligand. The water molecule has 2 lone electron
pairs, and chloride-ion has them four. However because the valence orbitals
in oxygen and chlorine are in sp3-hybridization state, and are located under
a corner of 10928 ’ they cannot overlap with the valence orbitals of the
same central ion, therefore such ligands act as monodentate. Exceptions are
polynuclear complexes, for example, Na2 [CuCl6]:
Cl
Na2
Cu
Cu
Cl
Cl
Cl
Cl
Cl
77
The nomenclature of complex compounds
The name of complex compounds are similar to the names of
simple salts and consists of the name of the cation and the anion. The
number and names of the ligands are included into the appropriate place:
[Cu (NH3) 4] Cl2 – tetra ammine copper(II) chloride;
K2 [Cu (OH) 4] – potassium tetra hydroxo cupprate(II);
Н [HgCl2] – hydrogen dichloromercurate(I);
[Cr (NH3) 3Cl3] – trichloro triammine chromium(III).
Classification of complex compounds
Different types of classification of complex compounds exists.
1. Based on the charge of inner sphere:
Cationic complexes: [Cr(H2O)6]Cl3, [Co(NH3)6]Cl3.
Anionic complexes: K2 [HgI4], Na[Sb(OH)6].
Neutral complexes: [Pt(NH3)2Cl2].
2. Based on the nature of the ligands:
Aqua-complexes with the water molecules as ligands:
[Cu(H2O)5]Cl2.
Ammine complexes with the ligands as molecules of ammonia or
organic ammines: [Cr(NH3)6]Cl3; [Cu(H2N-CH2-CH2-NH2)2]SO4.
Hydroxo complexes: K3[Al(OH)6].
Carbonyle complexes: [Fe(CO)5].
Acido-complexes with the acidic anions as ligands, can be further
devided into chloro complexes (K2[CuCl4]), cyano-complexes
(K3[Fe(CN)6), nitrito-complexes (K3[Co(NO2)6), etc.
3. Based on the nature of the central ion: complexes of copper,
silver, iron, chromium, etc.
Dissociation of complex compounds
and complex ions
The majority of complex compounds are electrolytes. In solutions
they are irreversibly dissociated into the inner and outer spheres (primary
dsociatiation):
78
[Cu(NH3)4] SO4 [Cu(NH3)4]2 + + SO42-
The complex ion is capable to the secondary dissociation as a weak
electrolyte (reversible and stepwise dissociation):
[Cu (NH3) 4] 2 +
[Cu (NH3) 3] 2 + + NH3
2+
[Cu (NH3) 3]
[Cu (NH3) 2] 2 + + NH3
2+
[Cu (NH3) 2]
[Cu (NH3)] 2 + + NH3
[Cu (NH3)] 2 +
Cu2 + + NH3
2+
2+
Total process: [Cu (NH3) 4]
Cu + 4NH3
Each of these reversible processes is characterised by an
equilibrium constant Кinst which carries the name of an instability constant
of a complex ion (stepwise or the overall):
K inst
[Cu 2 ][ NH 3 ] 4
2
[Cu( NH 3 ) 4 ]
The less the instability constant, the mire stable is the complex ion.
Instability constants are tabular values.
The equilibrium constant of the reaction of formation of a complex
ion at the expense of interaction of the central ion with the ligands carries
the name of a formation constant or the stability constant, Kf. As reaction
of formation of complex connection is opposite to its reaction
диссоциации,
Kf.= 1/Kinst
Shifting of the equilibrium of dissociation of a complex ion
submits to all rules of the le Chatelieu’s principle described previously.
Reactions of complex compounds
1. Formation of complex compounds
CuSO4 + 4NH3 [Cu(NH3)4]SO4
In the course of reaction, the complex ion is formed which is a
weak electrolyte, therefore the reaction is irreversible.
AgCl + 2NH3
[Ag(NH3)2]Cl
Weak electrolytess are present both among the reactants (insoluble
compound) and the products (a complex ion), therefore the reaction is
79
reversible. A direction of such reaction can be determined after calculation
of the equilibrium constant of the reaction.
2. Destruction of complex compounds
[Cu(NH3)4]SO4 + 4HNO3 CuSO4 + 4NH4NO3
(Complex ions are unstable in presence of strong acids and bases)
[Cu(NH3)4]SO4 + H2S
CuS + (NH4) 2SO4 + (NH4) 2S
3. Reactions of an exchange of the outer sphere
K[Sb(OH)6] + NaCl Na[Sb(OH)6] + KCl
(The reaction is possible because of formation of a precipitate)
4. Exchange reactions in the inner sphere
Ligands exchange:
K3 [Fe(SCN)6] + 6KCN
K3[Fe(CN)6] + 6KSCN
Central ion exchange:
K2 [SnCl4] + CuCl2
K2 [CuCl4] + SnCl2
(Кreaction = 5103, that means the reaction is possible)
Electronic structure of complex ions
Interaction of lone electronic pairs of the ligands with non-filled
valence orbitals of different types of the central ion leads to their
hybridization. For example, the electronic structure of an ion [Cu(NH3)4] 2+
can be reflected in the following way:
2 2
6 2
6
10 1
29Cu: 1s 2s 2p 3s 3p 3d 4s
2+
2 2
6 2
6
9 0
0
0
29Cu : 1s 2s 2p 3s 3p 3d 4s 4p 4d
3d
4s
4p
4d
4:NH3
Interaction of one s - and three p-orbitals leads to hybridization of
the sp3 type (tetrahedral complex).
Some valence d- and f-orbitals of the central ion may be occupied
by paired or unpaired electrons. This leads to their interaction with the lone
electron pairs of the ligands. This interaction is defined by the degree of
penetration of the lone pairs of the ligands into the d-subshell of the central
80
ion. From this poinf of view, the ligands may be arranged in a
spectrochemical series which allocate ligands of strong and weak fields:
CO,
CN
> NO2 -
> NH3
> SCN
-
> H2O
> OH -
-
> F-
> CL
-
Ligands of a strong field
Ligands of a weak field
Lone electron pairs of the ligands of a strong field deeply penetrate
into a valence electronic shell of the central ion and cause pairing of 3delectrons. As a result, inner-orbital low spin complexes are nformed
(complex compounds are formed with participation of internal 3d-orbitals,
the formed complexes have no small amount of non-paired electrons):
2 2
6 2
6
7 2
27Co: 1s 2s 2p 3s 3p 3d 4s
3+
2 2
6 2
6
6 0
0
0
27Co : 1s 2s 2p 3s 3p 3d 4s 4p 4d
3d
4s
4p
4d
4s
4p
4d
[Co (NH3) 6] 3 +
3d
6:NH3
Hybridization type is d2sp3, octahedral complex.
The lone electron pairs of the ligands of a weak field slightly
interact with the 3d-electrons of the central ion and do not cause their
pairing. Are as a result, outer-orbital high spin complexes are formed:
[CoF6] 3
3d
4s
4p
4d
6:F Hybridization type is sp d , octahedral complex.
3 2
81
82
PART 2
INORGANIC CHEMISTRY
83
84
S-ELEMENTS
(ALKALINE and ALKALINE EARTH METALS)
Alkaline metals Li, Na, K, Rb, Cs, Fr are situated in the IA group
of the periodic table. The outer spheres of these atoms consist of only one
electron. So atoms of alkaline metals have a tendency to lose their valence
electrons to be transformed into positively charged ions. Their oxidation
number is +1. The strength of the attraction of the outer electron to the
atom can be valued with the help of the ionization potential which
determines the energy of removal of one electron from a neutral atom. In
the IA group from Li to Fr the value of ionization potential decreases, the
chemical activity of metals increases. Alkaline metals are strong reducing
agents.
In air alkaline metals are easily oxidized, that is why they are
stored under oil.
Alkaline metals are more active than hydrogen (they have negative
values of redox-potentials) so they can replace hydrogen both from acids
and water:
2M + HCl = 2MCl + H2
2M + 2H2O = 2MOH + H2
In aqueous solutions metal hydroxides behave as strong
electrolytes and are fully dissociated:
MOH M+ + OH
Almost all salts of alkaline metals are water soluble. Solutions of
salts containing anions of weak acids undergo hydrolysis; they are basic.
Pure alkaline metals are produced by electrolysis of their melted
salts.
Biological significance of alkaline metals
Lithium is found in the liver and lungs of animals. Large
concentrations of lithium are dangerous for humans . Particles of dust and
smoke containing lithium provoke malignant tumors. Sodium as NaCl is
necessary for the balance of salt exchange in organisms, sodium
bicarbonate is used to lower the acidity of gastric juice. In living
85
organisms potassium is situated in liver, spleen, it regulates the function of
muscle cells and nervous systems. Rubidium is found in the leaves of
plants (beetroot, sugar-cane, tabacoo, tea, coffee, cocoa). In animal
organisms it is localized in muscles performing large load - heart muscle
and pectoral muscles of birds. Cesium is found in mineral water, plants and
living organisms. Compounds of rubidium and cesium are necessary for the
growth of plants.
Alkaline earth metals Ca, Sr, Ba, Ra are situated in the IIA group
of the periodic table. Atoms of these elements have two valence electrons.
While losing them, atoms of alkaline earth elements transfer into positively
charged ions and gain the oxidation number +2.
The presence of non-filled sublevels (d- and f-) makes Ca, Sr, Ba
and Ra more chemically active and having physical properties rather
different than those of Be and Mg.
Metals of IIA group are less active than alkaline metals. The
growth of the atomic radius, lowering of the ionization potential and of the
electronegativity define the growth of their chemical activity with increase
of charges of the nuclei.
In the air alkaline earth metals easily form oxides. They replace
hydrogen both from water and from acids:
M +2HCl = MCl2 + H2
M + 2H2O = M(OH)2 + H2
The basic character of oxides and hydroxides increases with the
increase of atomic radii from calcium to radium.
Alkaline earth metals form different sparingly soluble salts:
carbonates, phosphates, chromates, sulfates. The solubility of sulfates
lowers from calcium to radium.
Natural water containing soluble salts of calcium and magnum is
called hard water. The presence of hydrocarbonates of calcium and
magnum stipulates the temporary hardness of water. Chlorides and
sulfates of these elements cause the constant hardness. The sum of
temporary and constant hardness gives the overall hardness of water.
86
Biological and agricultural properties of elements of
IIA group
Beryllium and its compounds are toxic. The beryllium-poisoning
may cause the death.
Magnum compounds can be found in algae, fungi, ferns, in the
tissues of animals. Magnum is a complexing ion in chlorophyll.
Calcium is a constituent of the bones of vertebrates, predominantly
as ortophosphate Ca3(PO4)2. Egg-shells, tests of sea animals, shells mainly
consist of calcium carbonate. Organic salts of calcium play a significant
role in metabolism of plants. The deficiency in calcium leads to stopping
of their growth, development of rhizomes, the leaves cover by brown spots
and die off. The animals suffer from rachitis, the heart activity decreases,
the blood coagulability becomes worse.
Calcium ions enter human organisms with milk and meat meals
while magnum ions - with vegetable meal.
Strontium compounds in human organisms are mainly concentrated
in bones, the excess (more than 10-3 %) leads to their fragile.
There are approximately 100 times less barium compounds in
human bodies than those of strontium. In very small quantities barium
compounds stimulate activity of marrow. In large quantities they are very
toxic and provoke weakness, gastric-intestinal diseases, brain disorders.
Barium chloride and carbonate are used in the agriculture as chemical
weed-killers and pest-killers.
ELEMENTS OF IIIA AND IVA GROUPS
(P - ELEMENTS)
Boron and aluminum are elements of the IIIA group of the
periodic table. Atomic radius of boron is 0.91Å and the one of aluminum
is 1.43Å. This great difference affects chemical properties of these
elements. Ionization potential of boron is greater than that of aluminum.
87
Polarity of B-O chemical bond is small, so in solutions boron exists as
BO2 and BO33 ions (acidic properties). Al-O chemical bonds have a
more polar character, so in solutions aluminum exists both as Al 3+ and
AlO2 ions (amphoteric properties).
Salts of boric acid H3BO3 are metaborates (Ba(BO2)2) and
tetraborates (Na2B4O7 - borax).
Biological properties of boron and aluminum
Physiological activity of boron is rather high. Together with Mn,
Cu, Zn and Mo it is among five most important microelements. It
concentrates in bones, teeth, muscles, marrow, liver and thyroid gland, can
be found in adipose tissues of some animals, in milk and yolk of eggs.
Boron inhibits the action of amilaze and proteinaze, vitamins B2
and B12, reinforces the action of insuline. Boric acid and borax are used in
medicine as anticeptics.
Some compounds of aluminum are also used in medicine:
KAl(SO4)2 as astringent; AlOH(CH3COO)2 for desinfection; Al2(SO4)3 as
coagulant.
Carbon and silicon are elements of IVA group of the periodic table
of elements. Their highest oxidation number is +4.
At a room temperature carbon and silicon are inert elements, their
activities increase with heating. At high temperatures they react
with the majority of non-metals and metals.
Concentrated nitric and sulfuric acids oxidize carbon into CO2,
silicon can be oxidized by mixture of HNO3 and HF. Silicon can also be
dissolved in alkalis:
Si + 2NaOH Na2SiO3 + 2H2
Carbon (II) oxide CO is a non-salt forming oxide. Carbon dioxide
CO2 has acidic properties and reversibly dissolves in water to form a weak
carbonic acid:
88
CO2 + H2O
H2CO3
H+ + HCO3
Salts of carbonic acid are carbonates (Na2CO3) and
hydrocarbonates (NaHCO3).
In aqueous solutions carbonates and hydrocarbonates undergo
hydrolysis:
Na2CO3 + H2O
NaHCO3 + NaOH
Silicic acid is even weaker than carbonic acid, the reactions of
hydrolysis of its salts lead to the formation of polyanions:
2Na2SiO3 + H2O
Na2Si2O5 + 2NaOH
ELEMENTS OF VA AND VIA GROUPS
(P-ELEMENTS)
Nitrogen and phosphor are elements of VA group of the periodic
table. They have 5 electrons in the outer shell (valence electrons). They
may lose electrons and form positively charged ions (oxidation numbers
from +1 to +5) or gain electrons and form negatively charged ions
(oxidation number -3).
Hydrogen compounds of nitrogen and phosphor are ammonia NH3
and phosphine (PH3). The presence of a lone electron pair of nitrogen and
phosphor leads to the possibility of formation of a coordinate bond with a
proton. In aqueous solutions ammonia interacts with water molecules to
form ammonium hydroxide which possesses weak basic properties:
NH3 + H2O
NH4OH
NH4+ + OH
Ammonium ion has almost same properties as metallic ions, for
example it can form salts. Ammonium salts decompose while heating:
NH4Cl
NH3 + HCl
In oxides nitrogen has various oxidation states from +1 to +5.
Nitric acid HNO3 is one of the strongest acids with a high oxidizing
strength. Depending on the nature of a reducing agent and the concentration
89
of the acid, the NO3 group can gain from 1 to 8 electrons and transfer into
NO2, NO, N2O, N2 or NH4+.
Salts of nitric acid (nitrates) are water soluble.
Nitrous acid HNO2 is a weak acid with redox duality. It exists only
in diluted solutions and decomposes at high concentrations:
3HNO2 HNO3 + 2NO + H2O
In contrast with nitric acid, phosphoric acid has no oxidizing
properties. Phosphates form soluble complexes with a lot of metal ions.
Sulfur is situated in the VIA group of the periodic table and has 6
valence electrons. Its oxidation numbers are +4 , +6 and -2. Sulfuric acid
H2SO4 (conc.) is a strong oxidizing agent and hydrogen sulfide and its salts
(sulfides) are good reducing agents. Sulfurous acid H2SO3 and its salts
(sulfites) have redox duality.
The strength of acids containing sulfur increases with the increase
in oxidation number of sulfur.
Biological importance of sulfur
Sulfur is a composite of most important aminoacids. Metal sulfates
are used in medicine: CaSO4 as a stuff for plasters, BaSO4 in
rontgenoscopy of stomach, MgSO410H2O as purgative. Some antibiotics
have sulfur compounds as composites.
ELEMENTS OF THE VIIA GROUP
(HALOGENS)
Atoms of halogens have 7 electrons in the outer shell (ns2np5)
which determine their chemical activity. Halogens are strong oxidizing
agents. Their activities increase with decrease in ionic radii: fluorine is the
strongest oxidizing agent.
90
The properties of fluorine differ from those of other halogens. As
its atoms have no empty d-orbitals in the outer sphere, it can’t exist in
excited states, and its only oxidation number is -1.
Atoms of chlorine, bromine and iodine have a vacant d-orbital in
their outer spheres, so 3 electrons may be unpaired to form 3 excited states.
Possible oxidation numbers of these elements are -1, +1, +3, +5, +7.
Bond energies in the molecules of halogens increase with decrease
in atomic numbers: ECl-Cl > EBr-Br > EI-I.
ECl-Cl = 57.8 kcal/mol
EBr-Br = 46.1 kcal/mol
EI-I = 36.2 kcal/mol
The molecule of fluorine has the minimal bond energy which can
be explained by the features of electronic configuration of fluorine
comparing with other halogens.
Hydrogen compounds of halogens are colorless gases. The bond
energies decrease from HF to HI. Their aqueous solutions possess acidic
properties, HI is the strongest one among them. Reducing properties of
halogen hydrides increase with increase of charge of nuclei.
Oxygen containing compounds of halogens are strong oxidizing
agents.
Interactions of halogens with water can be expressed as:
F2 + H2O 2HF + [O]
[O] + F2 F2O
X2 + H2O HX + HOX (X = Cl, Br, I)
The equilibria of these interactions are shifted to the left. In
alkaline solutions the reactions become irreversible:
Cl2 + 2NaOH NaCl + NaClO + H2O
91
TRANSITIONAL ELEMENTS
(d-ELEMENTS)
d-Elements are situated in B-subgroups of the periodic table. Their
valence electrons are those of s-sublevel of the outer shell and of the
unfilled d-sublevel. The presence of 1 or 2 electrons on the outer shell of
all d-elements stipulates their metallic properties. d-Electrons take part in
the formation of chemical bonds, so different oxidation states are known
for d-elements. High values of oxidation numbers are typical only for delements with non-paired d-electrons (first 5 elements of each transitional
series). The values of a first ionization potential of d-elements of one
transitional series increases with increase in charge of the nuclei.
d-Elements in the same oxidation states usually have similar
properties. For example, all hydroxides of M(OH)2 and M(OH)3 types are
weak bases which are sparingly soluble in water. Sulfide and carbonate
ions form precipitates with M3+ and M2+ ions. All d-elements are good
complexing agents.
Ions of d-elements in their higher oxidation states possess acidic
properties and exist in solutions as anions: VO3 , CrO42 , Cr2O72 ,
MnO42 , MnO4 .
Biological significance of d-elements
All derivatives of Cr (VI) are strongly toxic and provoke ulcers and
lung cancer while breathing.
Manganese can be found in both animals and plants tissues.
Addition of small quantities of manganese to fertilizers increase the crop
capacity of some plants (maize, sugar-beet, potatoes and others).
Iron is a catalyst of respiratory processes. Human bodies contain
4g of iron, about 57% as haemoglobin. Soils contain from 1 to 5% of
iron compounds. Deficiency of iron provokes growing leaves pale.
Biological role of cobalt in living organisms is connected with
circulatory system. An antianaemic vitamin B12 contains 4.35% of cobalt.
Cobalt compounds also oppress malignant tumors.
92
Plants usually contain 10 4 % of zinc. Small amounts of zinc are
necessary for normal growth of animals and fruiting of plants.
Mercury and its compounds are very toxic and provoke disturbance
of cardiac and stomach activities and weakening of memory.
ELECTROCHEMICAL CORROSION OF METALS
The metals are good reducing agents. Their reducing ability is
described by the redox potential (Mn+/M0). The less the potential is, the
stronger are the reducing activities of the metal.
In the case of a macro-contact of two metals in presence of an
electrolyte (or their micro-contacts which take place in alloys), a process of
electrochemical corrosion, i.e. destruction of metals in the electrolyte
media, occurs. The stronger reducing metal (or a metal of a higer activity or
less (Mn+/M0) value) charges negatively because of the partial
transmission of the metal cations in the electrolyte medium:
M0 – n e Mn+
The liberated electrons move to the surface of a less active metal
and interact with the ions or molecules present in the electrolyte.
Depending on the pH of the medium, two processes are possible:
a) In acidic media (pH<7), the hydrogen depolarization occurs:
2H+ + 2 e H2;
b) In neutral (pH = 7) or in alkaline media (pH>7), the oxygen
depolarization becomes possible:
O2 + 2H2O + 4 e 4OHExample. Corrosion of a Cu/Fe contact in presence of NaOH.
(Cu2+/Cu0) = +0.34 V; (Fe2+/Fe0) = –0.44 V (Fe is more
active than Cu).
Fe – 2 e Fe2+
(x2)
Cu surface: O2 + 2H2O + 4 e 4OH - (x1)
2Fe + O2 + 2H2O 2Fe(OH)2
93
94
PART 3
ORGANIC CHEMISTRY
95
96
THE THEORY OF CHEMICAL STRUCTURE OF
ORGANIC COMPOUNDS
Organic compounds are carbon compounds (other than the
simplest) in which it exhibits a valence of IV.
Organic chemistry - a chemistry of hydrocarbons and their
derivatives.
The carbon atom in organic compounds is in an excited state, and
has four unpaired electrons :
2 2
2
2 1
3
→
6С 1s 2s 2p
6С* 1s 2s 2p
sp3
The carbon atom in the excited state is capable:
1) to form strong bonds with other atoms, which leads to the
formation of chains and cycles ;
2) due to the different types of orbital hybridization to form simple,
double and triple bonds between carbon and other atoms (H, O, N, S, P et
al.)
3) connect to four different atoms, which leads to the formation of
branched carbon chains .
Types of hybridization of carbon atoms in organic compounds
sp3 - hybridization
All four valence orbitals are involved in the hybridization. Valence
angle 109o28 '(tetrahedron). Carbon atoms form only simple (σ) bonds saturated compounds.
sp2 - hybridization
Three hybrid and one non-hybrid orbitals are formed. Bond angle
120° (flat structure, an equilateral triangle) . Hybrid orbitals form σ- bonds
and a non-hybrid orbital forms a π-bond.
97
sp - hybridization
Formation of two hybrid and two non-hybrid orbitals. Bond angle
of 180o (linear structure). The carbon atom in sp- hybridization takes part in
the formation of two double bonds or one triple bond.
The theory of the structure of organic compounds was formulated
by AM Butlerov in 1861 and includes the following provisions:
a)
All the atoms in the molecule are linked together in a strict
sequence according to their valences. The chemical structure determines
the way of connecting the atoms in a molecule.
b)
Properties of organic compounds depend not only on the
qualitative and quantitative composition of substances , but also on the
chemical structure of the molecule.
c)
Atoms in the molecule have a mutual influence on each
other, i.e., properties of atomic groups in the molecule may vary depending
on the nature of the other atoms in the molecule. A group of atoms that
determines the chemical properties of organic molecules is called a
functional group.
d)
Each organic compound having only one chemical formula.
Knowing the chemical formula, we can predict the properties of the
compound, and by studying its properties, in practice, to establish the
chemical formula.
organic molecule
carbon chain atoms
carbon skeleton
98
+
Functional Group identifies
the classes of organic
compounds
Types of the carbon skeleton :
- Acyclic :
• branched ;
• normal (linear) .
- Cyclic:
• carbocyclic (cycle of only carbon atoms);
• heterocyclic (except carbon atoms in the cycle includes several
other atoms - nitrogen , oxygen, sulfur) .
Types of carbon atoms in the hydrocarbon chain
СН3
Н3С-СН2-СН-С- СН3
СН3
-
СН3
Primary carbon atoms (connected in a chain with only one carbon
atom is the terminal);
Secondary carbon (bonded to two adjacent carbon atoms located
in the middle of the chain);
A tertiary carbon atom (located on the branched carbon chain
connected to three carbon atoms)
A tertiary carbon atom (located on the branched carbon chain
connected to three carbon atoms);
Functional group is a special group of atoms, which determines
the chemical properties of the compounds.
Examples of functional groups:
-ОН – hydroxyl group (alcohols, phenols);
С=О – carbonyl group (ketone, aldehyde);
ОН
С
- A carboxyl group (carboxylic acid);
О
99
-NH2 – Amino group (amine);
-SH – Thiol group (thioalcohols)
organic compound
Composition
Properties
chemical structure
The atoms that make up the organic compound can be connected in
different ways to form molecules. For example, a compound of C2H6O can
respond to two chemical compounds having different physical and
chemical properties:
H H
|
|
H C C O H
|
|
H H
H
H
|
|
H C O C H
|
|
H
H
ethanol
(b.p.= 78C,
Reacts with Na)
Dimethyl ether
b.p. = –24C,
does not react with Na)
Isomers are compounds having the same composition but different
chemical structure. Isomers have different chemical properties.
TYPES of ISOMERISM
1 structural isomers
Isomers of the carbon chain:
CH3 CH2 CH2 CH3
CH3 CH CH3
CH3
Butane
100
Methylpropane
Positional isomers of multiple bonds:
СН2=СН–СН2–СН3
СН3–СН=СН–СН3
Butene-1
Butene-2
Positional isomers of the functional group:
CH3 CH2 CH2 OH
CH3 CH CH3
OH
1-propanol
Interclass isomerism:
СН3–СН=СН–СН3
2-propanol
HC
CH
HC CH
butene-2
Cyclobutane
2-stereoisomers
Geometric (spatial, cis-trans isomers of compounds with double
bonds)
H
H
C
H3C
H
C
CH3
C
CH3
cis-butene-2
C
H3C
H
trans-butene-2
Geometrical isomerism is possible in the event that each of the
carbon atoms involved in the formation of a double bond, has a different
substituents. Thus, for a butene-1 CH2=CH-CH2-CH3, geometric isomerism
is not possible, since one of the carbon atoms at the double bond has two
identical substituents (hydrogen atoms).
Geometric (spatial, cis-trans isomers of cyclic compounds
101
H
H
CH3
H
H
H
H
CH3
H
CH3
H
CH3
cis- 1,2-dimethylcyclopropane
trans 1,2-dimethylcyclopropane
Geometrical isomerism is possible in the case when at least two
carbon atoms forming the ring, have different substituents
Optical isomerism:
Optical isomerism is stereoisomerism view caused the chirality of
molecules. In nature, there are compounds which correspond the two hands
of one person. One of the properties of these compounds is their
incompatibility with its mirror image. This property is called chirality
(from the Greek. "Sheir" the
hand).
The optical activity of molecules detected when exposed to
polarized light. If the solution through an optically active substance skip
polarized beam light, it will rotate the plane of polarization. Optical
isomers are designated using prefixes d- and l-.
For ease of reading optical isomerism Fischer proposed flat
formula reflecting the configuration of the chiral centers:
CHO
OH
CH2OH
D- glyceraldehyde
Mirrow
H
CHO
HO
H
CH2OH
L- glyceraldehyde
The optical isomerism is possible, if the molecule contains one or
more carbon atoms in sp3-hybridization condition that contain four
different substituents.
102
CONFORMATIONAL (SWING) ISOMERISM
As a result of the tetrahedral configuration of chemical bonds in the
hydrocarbon chains of saturated compounds are non-linear, and are
arranged in a zigzag pattern space; angle between the bonds corresponds to
the tetrahedral:
Free rotation occurs around a simple C-C bond, so the chain in
space can take many forms or conformations. Free rotation inhibited the
interaction of the hydrogen atoms on adjacent carbon atoms:
H
HH
H
H
H
H
H
H
eclipsed conformation
H
H
H
inhibited conformation
Conformational isomerism cycloalkanes linked with a nonplanar
structure of major cycles (containing more than 6 carbon atoms)
conformation "tub"
conformation "chair"
Homologous series
The special features of organic compounds can also be considered
as the existence of a homologous series, in which each successive term can
103
be generated from the previous addition of one specific for a given number
of groups of atoms. For example, in the homologous series of saturated
hydrocarbons such group is CH2. Homologues have similar chemical
properties, while the physical properties change somewhat with increasing
molar mass compounds:
СН4
Methane
С2Н6
ethane,
С3Н8
propane,
С4Н10
butane,
С5Н12
pentane
Types of Reactions in Organic Chemistry
Most organic compounds are characterized by a relatively low rate
of chemical reactions under normal conditions . This is due to the high
strength of the covalent bonds of carbon - carbon bond; Carbon - hydrogen;
carbon - oxygen and others.
Among the values of electronegativity carbon occupies an
intermediate position between the typical oxidants and reducing agents, so
that the difference in electronegativity and the polarity of carbon bonds
with other atoms is small (the chemical bonds in organic compounds of low
polarity and do not dissociate in solution , that is, the majority of organic
compounds - non-electrolytes) .
Types of fracture (splitting) of chemical bonds :
Homolytic decomposition of 1 - equivalent splitting the total
electron pair to form radicals :
СН4 → •СН3 + •Н
104
Stabilization of the formed radical is due to interaction with
neighboring carbon bonds. Radicals formed by the tertiary carbon atom
stable rest.
Heterolytic decay - unequal splitting of the total electron pair to
form carbocations and carbanions :
СН3Cl → СН3+ + Cl–
carbocation
СН3MgCl → СН3– + MgCl+
carboanion
Types of reactions in organic chemistry:
1 Substitution of (S).
2 Addition (A).
3 Splitting (elimination) (E).
4 Rearrangement (isomerization).
Attacking particles:
• radical (R).
• Negative and positive particles:
- Nucleophile (N) (-);
- An electrophile (E) (+).
HYDROCARBONS
Hydrocarbons are organic compounds that contain only carbon
and hydrogen atoms. Their characteristic feature is the lack of functional
groups. Properties of hydrocarbons determined by the structure of the
hydrocarbon radical.
Saturated hydrocarbons:
105
ALKANES
Alkanes (CnH2n+2, acyclic, all carbon atoms are in sp3-hybridization
condition).
Chemical properties. Low polar covalent bonds of alkanes and
very durable, so saturated hydrocarbons in chemical reactions are inactive.
The main types of chemical reactions of alkanes are:
1. Halogenation (radical substitution reaction, SR):
2 CH3 CH2 CH3 + Cl2
h
CH3 CHCl CH3 + CH3 CH2 CH2Cl + 2HCl
The formed alkyl halides are used for producing alkanes with a
longer carbon chain, as well as compounds of other classes (alkenes,
alcohols)
CH3
CH3
CH Br + 2 Na + Br
CH
CH3
CH3
CH3
to
2 NaBr + H3C CH CH CH3
CH3
(Wurtz reaction)
H2O
H3C CH CH3
+ NaBr
OH
H3C CH CH3 + NaOH
Br
o
t
C2H5OH
H3C CH CH2 + NaBr + H2O
2. Sulfochlorination.
RН + SO2 + Cl2 → HCl + RSO2Cl
RSO2Cl + NaOH → NaCl + RSO3Na (sodium alkyl sulfonate, based on
synthetic detergents)
3. Nitration (Konovalov’s reaction):
106
t, P
CH3 CH2 CH3 + HNO3
CH3 CH CH3
+ H2O
NO2
2-nitropropane
3. Oxidation
СnH2n+2 + (1,5n+0,5)O2 nCO2 + (n+1)H2O
(burning);
4. Isomerization
CH3 CH2 CH2 CH3
o
100 C , AlCl3
CH3 CH CH3
CH3
5. Dehydrogenation:
CH3 CH2 CH2 CH3
o
300-450 C
CH3 CH CH CH3 + H2
Al2O3 или Cr2O3
CH3 CH2 CH2 CH3
o
400-600 C
Al2O3
CH2 CH CH2 CH3 + H2
CH2 CH CH CH2 + 2 H2
CH3 CH2 CH2 CH2 CH2 CH3
o
300 C
Pt
+ 4H2
CH3 CH2 CH2 CH2 CH2 CH2 CH3
o
300 C
Pt
CH3
+ 4H2
6. Cracking:
СH4
1000oC
C + 2H2;
С18H38
2CH4
t>1500oC
C2H2 + 3H2;
t
C9H18 + C9H20
107
CYCLOALKANES
Cycloalkanes (CnH2n are cyclic, all the carbon atoms are in sp3hybridization).
The angles between the bonds in small rings - C3H6 (600) and C4H8
0
(90 ) are less than required by the geometry of the carbon atom in the state
of sp3-hybridization (109.50), so they are unstable, and characterized by the
addition reaction, coming from breaking up the cycle:
С4H8 + H2
С3H6 + HBr
Pd, t
C4H10;
t
C3H7Br;
С3H6 + Br2
t
C3H6Br2.
Cycloalkanes based on 5, 6 and 7 carbon atoms (normal cycle) are
chemically inactive, same as alkanes, they are characterized typical
substitution reaction
С6H12 + Сl2
h
C6H11Cl + HCl.
Unsaturated hydrocarbons
ALKENES
Alkenes (CnH2n are acyclic compounds with thecarbon atoms in the
states of sp3- and sp2 hybridization with one double bond).
Addition reactions (in electrophilic mechanism, AE)
1. Halogenation:
CH2=CH2 + Br2 CH2Br–CH2Br
(discoloration of bromine water - an aqueous solution of bromine is a
qualitative reaction of compounds with double and triple bonds).
When the temperature rises above 500 ° C in the halogenation of alkenes
are substitution reaction
108
СН3-СН=СН2 + Br2
540C
СН3-СBr=СН2 + HBr
2. Hydrogenation and dehydrogenation:
CH2=CH2 + H2
CH2=CH–СН2–СН3
, Pt , Pd ил и Ni
t
CH3–CH3
, Cr2O3
t
CH2=CH–СН=СН2
3. Hydrohalogenation:
СH3–CH=CH2 + HBr CH3–CHBr–CH3
(the reaction proceeds via Markovnikov's rule: hydrogen is added to
the double bond to the most hydrogenated carbon atom).
In the presence of hydrogen peroxide or other peroxide compounds
reaction mechanism is changed to a radical reaction and
gidrogalogenirovaniya goes against the rules of Markovnikov (Kharasch
effect):
Н2О2
СН3-СН=СН2 + HCl
СН3-СН2-СН2Cl (АR)
4. Hydration:
СH3–CH=CH2+H2О
3 PO4
H
CH3–CH(ОН)–CH3
Oxidation reactions
СnH2n + 1,5nO2 nCO2 + nH2O (burning);
3CH2=CH2 + 2KMnO4 + 4H2O 3HO–CH2–CH2–OH + 2MnO2 +
2KOH
(soft oxidation in a neutral medium);
5СH3–CH=CH–CH3 + 8KMnO4 + 12H2SO4
10CH3COOH + 8MnSO4 + 4K2SO4 + 12H2O;
5 CH 3 C CH 2 + 8KMnO4 + 12H2SO4
|
CH3
109
5 CH 3 C O + 5CO2 + 8MnSO4 + 4K2SO4 + 17H2O
|
CH3
(hard oxidation in acidic medium).
Polymerization
n(CH3–СH=CH2)
p, t, катализатор
CH CH 2
|
CH
3
n
Polymer is a macromolecule consisting of a large number of
recurring units called monomers. The number of repetitions of the
monomers in the chain (n) is called the "degree of polymerization"). In the
polymerization process, a mixture of macromolecules with different
degrees of polymerization is usually obtained, so the polymers are not
characterized by a fixed melting point, and melt in the temperature range.
The polymerization reactions associated with rupture of the
double bonds in the molecules of monomers.
ALKADIENES
Alkadienes (CnH2n-2 are acyclic. Their carbon atoms are in the sp2
and sp -hybridization states with two double bonds). Only conjugated
dienes (1,3-dienes) are of biological significance
3
110
Dienes having various substituents on the carbon atoms at the
double bond, like alkenes exhibit cis-trans isomerism.
The chemical properties of conjugated alkadienes
Electrophilic addition reaction and the polymerization of
conjugated alkadiene occur, usually at positions 1 and 4:
CH2=CH–CH=CH2 + Br2 CH2Br–CH=CH–CH2Br
CH2Br–CH=CH–CH2Br + Br2 CH2Br–CHBr–CHBr–CH2Br
CH2=CH–CH=CH2 + 2Br2 CH2Br–CHBr–CHBr–CH2Br;
CH2=CH–CH=CH2 + HBr CH3–CH=CH–CH2Br
CH3–CH=CH–CH2Br + HBr CH3–CHBr–CH2-–CH2Br
CH2=CH–CH=CH2 + 2HBr CH3–CHBr–CH2-–CH2Br.
Hydration is not characteristic of conjugated alkadienes, as by
heating in presence of acids (condition for hydration of alkenes) their
polymerization occurs, which leads to the formation of rubbers:
p, t, катализатор
nCH2=CH–CH=CH2
(–CH2–CH=CH–CH2–)n
ALKYNES
Alkynes (cnH2n-2 acyclic carbon atoms are in the states sp and sp3hybridization within the molecule contains one triple bond).
Addition reactions
Ni , t
Hydrogenation:
СHCH + 2H2
CH3–CH3
Halogenation CHCH + Br2 CHBr=CHBr;
111
CHBr=CHBr + Br2 CHBr2–CHBr2
or
CHCH + 2Br2 CHBr2–CHBr2
Hydrohalogenation:
CHCH + НBr CH2=CHBr;
CH3–ССН + HBr СH3–CBr=CH2;
CH3–CBr=CH2 + HBr CH3–CBr2–CH3
Hydration (Kucherov reaction):
HC
CH + H2O
HgCl2
O
[H2C CH]
H3C
C
H
OH
HC
C CH3 + H2O
HgCl2
[H2C CH CH3]
H3C
OH
C CH3
O
Polymerization
СН2=СН–CCH
2СНСН
CuCl , NH 4Cl
3CH CH
o
600 C, C активир.
(benzene);
Acidic properties of alkynes
Terminal alkynes have acidic properties - the substitution of the hydrogen
atoms have sp-hybridized carbon atom of the metal:
СНСН + 2[Ag(NH3)2]OH Ag–CC–Ag + 4NH3 + 2H2O;
CH3–CCH + CuCl + NH4OH CH3–CC–Cu + 2NH3 + H2O;
CH3–CC–CH3 + Ag2O (NH3)
CH3–CCH + NaH CH3–CC–Na + H2;
CH3–CC–Na + H2O CH3–CCH + NaOH
CH3–CC–Na + СH3–CH2I NaI + CH3–CC–CH2–CH3
112
AROMATIC HYDROCARBONS
Aromatic hydrocarbons or arenes (CnH2n-6 are cycliccompounds
with the carbon atoms in sp2-hybridization state, a molecule contains
conjugated system of double bonds).
H
HC C C H
C
C
H C H
H
или
The representatives of the homologic series of arene are:
С6Н6
С7Н8
CH3
benzene,
methylbenzene (toluene)
С8Н10
CH2 CH3
CH3
ethylbenzene
CH3
CH3
CH3
H3C
CH3
1,21,31,4dimethylbenzene dimethylbenzene dimethylbenzene
(o-xylene)
(meta-xylene)
(p-xylene)
CH CH2
vinylbenzene (styrene)
H3C CH CH3
isopropylbenzene (cumene)
113
In condensed aromatics two adjacent "spliced" cycle have two
atoms in common. Thus, three types of coupling loops.
naphthalene
anthracene
phenanthrene
Chrysene
pyrene
Benzpyrene
Chemical properties . Conjugated π- electron system of benzene
and its homologues is an energetically favorable state, so its destruction
occurs with great difficulty. Addition reaction to arenes are not typical,
more common are electrophilic substitution reactions (SE). They occur in
presence of catalysts : Fe, trivalent metal salts - Lewis acids.
Lewis acids are particles that are capable of accepting lone electron
pairs:
AlCl3 + Cl– → [AlCl4]–
Chemical properties of benzene
1. Halogenation:
114
+ Br2
Br
AlBr3 или FeBr3
+ HBr
2. Nitration
NO2
H2SO4 (конц.)
+ HNO3 (HO NO2)
+ H2O
.
3.Alkylation
CH3
AlCl3
+ CH3Cl
+ HCl
;
+ CH2 CH CH3
CH CH3
H3PO4
CH3
;
CH CH3
H2SO4
+ CH3 CH CH3
CH3
+ H2O
OH
.
4. Addition reactions:
+ 3 H2
P, t, Ni
(cyclohexane);
H
+ 3 Cl2
h
Cl
H
Cl
H
Cl
Cl
H
Cl
H Cl
H
(hexachlorocyclohexane).
115
5. Oxidation: 12С6Н6 + 15О2 12СО2 + 6Н2О (burning). Benzene can
not be oxidized by potassium permanganate.
Chemical properties of benzene homologues
The alkyl substituents on the benzene ring slightly increase the
activity of the benzene ring and activy the substitution at positions 2, 4, and
6 relative to the alkyl substituent (ortho and para-substitution)
CH3
Br
CH3
+ HBr
+ Br2
AlBr3 или FeBr3
CH3
+ HBr
Br
Nitration of toluene leads to the formation of the trinitro-substituent:
CH3
CH3
H2SO4
O2N
NO2
+ 3 H2O
+ 3 HNO3
NO2
(trinitrotoluene).
When irradiated with the UV light, the benzene homologues
undergo the reaction of substitution in the side chain, with the greatest
activity are carbon atoms directly connected to the benzene ring:
CH2 CH2 CH3
+ Br2
CH CH2 CH3
h
Br
+ HBr
.
116
Benzene homologues burn to form carbon dioxide and water, and
can be oxidized with potassium permanganate in the side chain to form a
benzoic acid or its salts:
С6Н5СН3 + 9О2 7СО2 + 4Н2О (burning);
5С6Н5CH3 + 6KMnO4 + 9H2SO4
5C6H5COOH + 6MnSO4 + 3K2SO4 + 14H2O;
t
С6Н5CH3 + 2KMnO4
C6H5COOK + 2MnO2 + KOH + H2O;
5С6Н5CH2CH3 + 12KMnO4 + 18H2SO4
5C6H5COOH + 5CO2 + 12MnSO4 + 6K2SO4 + 28H2O;
t
С6Н5CH2CH3 + 4KMnO4
C6H5COOK + K2CO3 + 4MnO2 + KOH + 2H2O.
Characteristics of the chemical properties of condensed rings
The mutual influence of the two condensed rings (e.g., in
naphthalene) leads to increased activity of the hydrogen atoms in the
α-position:
Br
Br2
HBr
117
KMnO4
COOH
COOH
ORGANIC COMPOUNDS WITH FUNCTIONAL
GROUPS
Functional group is a group, which defines the affiliation to a
class of organic compounds and chemical properties. Depending on the
number and nature of functional groups, the following types can be
distinguished:
Monofunctional compounds which have only one functional
group of alcohols, aldehydes, ketones, carboxylic acids, ethers, esters,
phenols, amines, etc.
Multifunctional compounds that have multiple identical functional
groups (polyhydric alcohols, polybasic acids and others.).
Polyfunctional compounds that contain several different types of
functional groups: amino acids, carbohydrates, etc.
AMINES
Amines are organic derivatives of ammonia in which one or more
hydrogen atoms are replaced by a hydrocarbon radical:
118
H3C
CH3–NH2
CH3–NH–CH2–CH3
methylamine
(primary amine)
methylethylamine
(secondary amine)
N CH3
H3C
trimethylamine
(tertiary amine)
In the aromatic amines, the nitrogen atom is connected to the
benzene ring:
NH2
H
CH3
N
H 3C
CH3
N
N
Phenylamine
(Aniline)
Methylphenylamine Dimethylphenylaminee Triphenylamine
(N-methylaniline)
(N, Ndimethylaniline)
Chemical properties
1: Basic properties. The nitrogen atom in the ammonia and amines
has an lone electron pair able to bind hydrogen ions due to the donoracceptor interactions. CH3–NH2 + Н2О CH3–NH3+ ОН–
methylamine
methylammonium hydroxide
CH3–NH2 + НСl CH3–NH3+ Cl–
Saturated amines are stronger bases than ammonia; secondary
amines are stronger than the primary ones. Amine salts are decomposed by
the action of strong inorganic bases:
CH3–NH3Cl + NaOH CH3–NH2 + NaСl + H2O.
119
Aromatic amines are much weaker than ammonia and aliphatic
amines. This is due to the fact that the lone electron pair of the nitrogen
atom is partially shifted to the aromatic ring by conjugation with the πelectrons of the benzene rings and are therefore less available for
interaction with the proton:
С6H5NH2 + H2O ;
C6H5NH2 + HCl C6H5NH3+ Cl–
aniline
phenylammonium chloride
2. Reactions of burning. Combustion of amines is not
accompanied with the formation of nitrogen oxides but nitrogen as a simple
substance:
4CH3NH2 + 9O2 4CO2 + 10H2O + 2N2 or
CnH2n+3N + (1,5n+0,75)O2 nCO2 + (n+1,5)H2O + 0,5N2.
3. Interaction of the amino group of aromatic amines with the
benzene ring. The amino group facilitates the electrophilic substitution
reaction of hydrogen atoms on the benzene ring and orients them in the
ortho and para positions. Aniline discolours the bromine water. At the same
time, a white precipitate isd formed. This is a qualitative reaction for
aromatic amines:
NH2
NH2
Br
Br
+ 3Br2
+ 3HBr
Br
ALCOHOLS
Alcohols are organic compounds containing one or more hydroxyl
groups (OH) attached to a hydrocarbon radical.
120
Monohydric saturated alcohols
Monohydric alcohols contain one hydroxyl group bonded to a
saturated hydrocarbon radical. The general formula : R-OH, CnH2n+1OH.
For alcohols, regioisomers of the functional group and isomers of the
carbon skeleton are known.
Alcohols in which the hydroxyl group is in the 1st position
(attached to a primary carbon atom), are called the primary alcohols. If the
hydroxyl group is located in the middle of the chain (attached to a
secondary carbon atom), it is called a secondary alcohol. Joining hydroxyl
group to a tertiary carbon atom results in the formation of tertiary alcohols.
primary
secondary
tertiary
alcohols
While naming alcohols, the position of the OH group and the end
“ol” are used:
CH3 OH
CH3 CH2 OH
CH3 CH2 CH2 OH
methanol
ethanol
1-propanol
CH3 CH CH3 CH3 CH2 CH2 CH2 OH
OH
2-propanol
1-butanol
CH3 CH CH2 OH
CH3
1-methylpropanol
121
CH3 CH CH2 CH3
CH3
CH3
OH
C CH3
OH
2-butanol
2-methylpropanol
Chemical properties.
Characteristics of the chemical properties of alcohols are defined
by the presence of a functional group OH. Electronegative oxygen atom
pulls on shared electron pairs, thus acquiring a negative charge, and the
neighboring carbon and hydrogen atoms produce positive charges.
Displacement of the electron pairs leads to the formation of polar bonds
which are less stable and more reactive .
Reactions involving the cleavage of the RО–Н bond
а).
2С2H5OH + 2Na H2+ 2C2H5ONa
sodium ethoxide
С2H5ONa + Н2O С2H5OH + NaOH
(hydrolysis of alkoxides);
С2H5OH + NaОН
(monohydric alcohols do not react with alkalis).
b). Formation of esters:
С2H5OH + СН3СООН
H+, t
Н2О + СН3СООС2Н5
(ethyl acetate);
С2H5OH + HNO3 (HO–NO2)
Н2О + С2Н5ОNO2.
122
Reactions involving the cleavage of the R–ОН bond
С2H5OH + HCl C2H5Cl + H2O;
а).
b). Intramolecular dehydration:
t 180o C , H 2 SO4
СH3–СН2–OH H2O + СН2=СН2
180o C , H 2 SO4
СH3–СН2–СН(OH)–СН3 t
СH3–СН=СН–СH3 + H2O
(reaction proceeds according to the Zaitsev’s rule: the H atom is detouched
from the least hydrogenated carbon atom).
c). Intermolecular dehydration (formation of ethers)
CH3 CH2 OH t 140o C , H 2 SO4
H2O + CH3 CH2 O CH2 CH3
CH3 CH2 OH
(dimethyl ether).
Ethers form a separate class of organic compounds isomeric to
saturated monohydric alcohols (interclass isomerism).
Oxidation of monohydric alcohols
a). burning
СnH2n+2O + (1,5n +0,5)O2 nCO2 + (n +1)H2O.
b). Air oxidation in the presence of catalysts:
CH3 CH2 OH
300 500 o C , Cu
CH3
O
C
+ H2
H
(primary alcohol)
(aldehyde)
500 C , Cu
300
o
CH3 CH CH3
OH
CH3 C CH3 + H2
O
123
(secondary alcohol)
(ketone)
c). Oxidation by copper oxide (II):
CH3 CH2 OH + CuO
t
CH3
O
C
H
(primary alcohol)
+ H2O
(aldehyde)
d). Oxidation with potassium permanganate or potassium dichromate at
room temperature (primary alcohols are oxidized to carboxylic acids,
secondary alcohols - to ketones, tertiary alcohols are not oxidized under
these conditions)
5СH3OH+6KMnO4+9H2SO4 5CO2+6MnSO4+3K2SO4+19H2O;
5СH3–СН(OH)–СН3+2KMnO4+3H2SO4
5СH3–СO–СН3+2MnSO4+K2SO4+8H2O.
Monohydric unsaturated alcohols
HC C CH2
1-propenol
(allyl alcohol)
OH
1-propynol
(propargyl alcohol)
3-phenyl-1-propenol
(cinnamic alcohol)
Structural isomers of unsaturated alcohols are related to the
structure of the carbon chain and the positions of multiple bond and a
hydroxyl group. The multiple bond and OH groups can not be attached to
the same C atom because of the isomerization into aldehydes or ketones:
O
CH2
CH OH
CH3 C
H
vinyl alcohol
124
ethanal
.
Unsaturated alcohols possesst the properties of both alcohols and
unsaturated compounds.
Polyhydric alcohols (polyols)
Polyhydric alcohols contain several hydroxyl groups attached to
different carbon atoms. Attaching multiple hydroxyl groups to the same
carbon atom is impossible, since the dehydration process occurs:
O
OH
CH3
CH
OH
CH3
OH
C OH
OH
H2O + CH3
C
H
O
H2O + CH3
C
OH
Examples of polyhydric alcohols:
H2C
CH2
HO OH
ethanediol (ethylene glycol)
CH2
CH CH2
OH
OH OH
diatomic propantriol (glycerol)
CH2
*
*
CH CH CH CH2
CH2
*
*
*
*
CH CH CH CH CH2
OH
OH OH OH OH
OH
OH OH OH OH OH
xylitol
sorbitol
Chemical properties of polyols
1. Acidic properties
CH2 OH
+ 2Na
CH2 OH
CH2 ONa
CH2 ONa
+ H2 ;
125
CH2 OH
CH2 OH
CH2 ONa
+ NaOH
CH2 OH
2 Qualitative reaction to polyols:
CH2 OH
_ CH2 OH
OH
CH O
2 CH OH + Cu(OH)2
Cu
CH2 OH
CH2 O
H
blue precipitate
+ H2O .
(glycolate)
H
O CH2 + 2H O
2
O CH
HO CH2
dark blue solution (Cu glycerate)
3 Formation of full and partial esters with inorganic or organic
acids:
CH2 OH
CH OH
CH2 O CO CH3
+ 3CH3COOH
CH2 OH
CH2 O CO CH3
CH2 OH
CH OH
CH2 OH
CH2 OH
+ 3HNO3
(HO NO2)
+ H3PO4
(HO PO3H2)
4. Dehydration of polyols
126
;
CH2 O NO2
CH2 OH
CH OH
CH O CO CH3 + 3H2O
CH O NO2
+ 3H2O
CH2 O NO2
(nitroglycerin)
CH2 OH
CH OH
CH2 O
+ H2O
PO3H2
.
(dioxane)
PHENOLS
Phenols are aromatic organic compounds in which the hydroxyl
groups of the molecules are linked to carbon atoms of the aromatic ring:
OH
OH
OH
OH
CH3
CH3
phenol
ortho-cresol
meta-cresol
CH2OH
benzyl alcohol
(does not possess phenolic
properties)
OH
CH3
para-cresol
OH
α-naphthol
OH
β-naphthol
OH
OH
OH
OH
OH
catechol
resorcinol
hydroquinone
127
OH
OH
OH
HO
OH
OH
HO
OH
OH
pyrogallol
phloroglucinol
oxyhydroquinone
Chemical properties of phenols
1. Reactions of the OH group
a) Acidic properties
2C6H5OH + 2Na H2 + 2C6H5ONa (sodium phenolate);
C6H5OH + NaOH C6H5ONa + H2O;
C6H5ONa + H2O + CO2 C6H5OH + NaHCO3
(acidic properties to phenol are less than that of carbonic acid);
ONa
OH
OH
+ CO2
COONa H+/H O
2
125oC
COOH
6 àòì
ô åí î ëÿò
í àòðèÿ
ñàëèöèëî âàÿ
êèñëî òà
ñàëèöèëàò
í àòðèÿ
sodium phenolate sodium salycilate
salycilic acid
_
e
2 C6H5OH + FeCl3 + 4 H2O
_
+3
[Fe(H2O)4 C6H5OH C6H5O]Cl2 + HCl
+2
[Fe(H2O)4 C6H5OH C6H5O]Cl2
ô èî ëåòî âû é
Violet staining solutions in the presence of iron chloride (III) is a
qualitative reaction on phenols.
128
b) Formation of ethers and esters
С6Н5ОН + СН3СООН
O
C6H5ONa + CH3 C
Cl
O
CH3 C
O C6H5
+ NaCl
C6H5ONa + R–Br C6H5OR + NaBr
c) Oxidation
OH
O
OH
[O]
O
[O]
O
OH
O
benzoquinone
1. Reactions of the benzene ring (similar to the aromatic
amines):
Br
Br
+ r2
+ 3 HBr
Br
O2N
NO2
+ HNO3
+ 3 H2O
NO2
129
Trinitrophenol (picric acid) is a yellow crystalline solid, the acidic
strength approaches inorganic acids).
Polycondensation with formaldehyde (resins formation):
OH
OH
O кислотные или щелочные
катализаторы
+nH C
H
(n+1)
CH2
OH
CH2
+ n H2O
n-1
ALDEHYDES AND KETONES
Aldehydes and ketones are carbonyl containing compounds:
O
C
.
O
H C
H
methanal
(formaldehyde or
formic aldehyde)
C
CH2
CH2
ethanal
(acetaldehyde or
acetaldehyde)
C
H
butanal
(butyraldehyde)
CH3 C CH3
O
propanone (acetone)
CH2
C
CH
C
H
O
CH2
methylpropanal
(Isobutyraldehyde)
CH3
CH2 C CH3
O
butanone
H
propanal
(propionaldehyde)
O
CH3
CH3
130
CH3
H
O
CH3
O
O
CH3
CH
C
H
propenal
(acrolein)
CH3
CH2
CH2 C CH3
O
2-pentanone
CH3
CH2 C CH2 CH3
CH3 C C6H5
CH3
O
CH3
CH C CH3
O
O
3-pentanone
C6H5 C
methylbutanone
O
methylphenylketone
(acetophenone)
C6H5
C C6H5
O
benzaldehyde
diphenyl ketone (benzophenone)
Chemical properties
H
Carbon atom of the carbonyl group is in the sp2-hybridization (flat
fragment). Electrons of the double bond are strongly biased toward the
more electronegative oxygen atom (C = O bond polar). Redistribution of
charges in the carbonyl group has an effect on the polarity of the C-H
bonds adjacent to the carbonyl group carbon atom (-position):
H
R C
+
C
_
O
H
H
a. Addition reactions involving C = O groups (nucleophilic
addition SN)
CH3 C
O
H
X
CH3 CH
X
a) Addition of hydrogen (reduction of aldehydes and ketones to
primary and secondary alcohols)
131
O
CH3 C
t, Ni
+ H2
CH3 CH2 OH
H
t, Ni
CH3 C CH3 + H2
CH3 CH CH3
OH
O
b) Addition of alcohols
CH3 C
O
+ HOR
H
CH3 CH OH
OR
(hemiacetal);
OH
CH3 C CH3
CH3 C CH3 + HOR
OR
O
(acetal).
c) Addition of sodium hydrosulfite (the reaction is used for
separation of aldehydes and ketones from mixtures with other organic
substances):
O
CH3 C
+ NaHSO3
H (HO-SO Na)
2
CH3 CH OH
OSO2Na
OH
CH3 C CH3
CH3 C CH3 + NaHSO3
(HO-SO2Na)
O
OSO2Na.
d) Addition of ammonia and ammonia derivatives
CH3
CH3
CH3
C CH3 + H
O
132
NHR
CH3
C NH R
OH
_
CH3 C N R
H2O àçî ì åòèí û
(î ñí î âàí èÿ Ø èô ô à)
azometines (Shiff bases)
O
H
OH
+ HN NH2
H3C C
H3C CH NH NH2 _
H
H2O
H3C CH N NH2
ãèäðàçî í
hydrazone
2. Substitution reactions at -carbon atom
O
H3C C
O
+ 3 Cl2
Cl3C C
_
3 HCl
H
H
Iodoform test is a qualitative reaction for a carbonyl group:
O
H3C C
I2, NaOH
CHI3 + RCOONa
èî äî ô î ðì
(æåëòû é î ñàäî ê)
R
Iodoform (yellow precipitate)
Since the primary and secondary alcohols can be oxidized by
iodine to aldehydes and ketones, they also show the Iodoform test:
H3C CH2 OH
I2, NaOH
CHI3 + HCOONa
3. Condensation reactions of aldehydes and ketones
a) Aldol condensation:
O
H
O
+ H2C C
H3C C
H
OH
H3C CH CH2 C
H
O
H
133
CH3
H3C C CH2 C CH3
H3C C CH3 + H2C C CH3
O
OH
H O
O
b) Crotonic condensation:
CH3
CH3
to
H3C C CH2 C CH3 _
H3C C CH C CH3
H2O
OH
O
O
H3C C CH3 + H2C C CH3
O
H O
4. Oxidation of aldehydes
a) Silver mirror reaction (qualitative reaction for aldehydes):
O
CH3 C
Ag2O (4NH3)
t
O
CH3 C
H или 2[Ag(NH ) ]OH
3 2
(аммиачный раствор оксида серебра)
Ag + 3NH3
ONH4 (металлический налет
на стенках пробирки)
c) Reaction with copper(II) hydroxide (qualitative reaction for
aldehydes):
O
t
CH3 C 2Cu(OH)2
H (голубой осадок)
O
H C 4[Ag(NH3)2]OH
H
t
O
H C 4Cu(OH)2
H
134
O
CH3 C
Cu2O
OH (красный осадок)
(NH4)2CO3 Ag + 6NH3
t
CO2 2Cu2O 5
Ketones can not be oxidized under the given conditions.
CARBOXYLIC ACIDS
Carboxylic acids compose a class of organic compounds containing
a carboxyl functional group
O .
C
OH
In its structure, the carboxyl group can be associated with only one
carbon atom (end group), so the isomerism of carboxylic acids is associated
with the isomerism of the carbon skeleton or position of multiple bonds.
Classification and nomenclature of carboxylic acids
1. Saturated monocarboxylic acids (general formula СnH2n+1COOH
or CnH2nO2).
Formula
Н–СООН
СН3–СООН
СН3–СН2–СООН
СН3–СН2–СН2–СООН
CH3 CH COOH
CH3
СН3–СН2–СН2–СН2–СООН
С15Н31СООН
С17Н35СООН
Name (common system)
methanoic (formic)
ethanoic (acetic)
propanoic
butiric
isobutiric
valeric
palmitic
stearic
135
2. Unsaturated monocarboxylic acids
(general formula СnH2n–1COOH or CnH2n–2O2)
Formula
Name (common system)
СН2=СН–СООН
acrylic
CH2 C COOH
metacrylic
CH3
СН3–СН=СН–СООН
С17Н33СООН
crotonic
oleic
С17Н31СООН
lynolic
C O
OH
С17Н29СООН
lynolenic
C O
OH
С19Н31СООН
araquidonic
C O
OH
3. Saturated dicarboxylic acids
Formula
Name (common system)
НООС–СООН
Ethanadioic (oxalic)
НООС– СН2–СООН
Propanedioic (malonic)
НООС– (СН2)2–СООН
Butanedioic (succinic)
НООС– (СН2)3–СООН
Pentanedioic (glutaric)
НООС– (СН2)4–СООН
Hexanedioic (adipic)
4. Unsaturated dicarboxylic acids
136
H
H
H
C C
HOOC
COOH
Maleic acid (cis-isomer)
5. Aromatic carboxylic acids
Formula
COOH
C C
HOOC
H
Fumaric acid (trans-isomer)
Name
COOH
benzoic
COOH
COOH
phthalic
COOH
isophthalic
COOH
COOH
terephthalic
COOH
Polyfunctional acids
6. Hydroxyacids
Formula
*
CH3 CH
COOH
Name
lactic
OH
137
malic
*
CH2 CH COOH
HOOC
OH
OH
HOOC
citric
C CH2 COOH
CH2 COOH
tartaric
*
*
HOOC CH CH COOH
OH OH
salicylic
COOH
OH
Oxyacids usually possess optic activity:
COOH
H
Mirror
COOH
OH
CH3
D- lactic acid
OH
HO
H
COOH
D-tartaric
H
CH3
L- lactic acid
COOH
H
HO
COOH
HO
H
COOH
H
H
OH
OH
H
OH
COOH
L-tartaric
COOH
mesotartaric
Optically
inactive
because of the inner
138
symmetry plane
Chemical properties
The structure of the carboxyl group
O
H
R C
D
H
C
C
B
A
O
H
The reaction centers in the molecules of carboxylic acids are:
A. OH of the carboxyl group.
B. C-OH of the carboxyl group.
C. R-COOH.
D. C-H bond of the hydrogen atom in the -position
A. Dissociatopn of the COOH group:
RCOOH
RCOO– + H+
(carboxylic acids are weak acids)
The C-O bonds in the formed carboxylate anion are aligned, the
negative charge is evenly distributed between the two oxygen atoms,
therefore, the double C = O bond in carboxylic acids is inactive.:
O
_
R
C
O
Acidic properties of carboxylic acids:
2СH3COOH + Fe (СH3COO)2Fe + H2;
2CH3COOН + MgO (CH3COO)2Mg + H2O;
CH3COOH + KOH CH3COOK + H2O;
139
CH3COOH + NaHCO3 CH3COONa + CO2 + H2O.
B. Elimination of the hydroxyl radical OH (nucleophilic
substitution in carboxylic acids, acylation reaction)
Esterification:
O
CH3 C
+ HO CH3
O
H2SO4(конц.)
CH3 C
OH
+ H2O
O CH3
(methyl acetate)
Formation of acid halides:
O
O
+ PCl5
CH3 C
CH3 C
+ POCl3 + HCl
Cl
OH
Acetyl chloride
O
O
+ SOCl2
CH3 C
CH3 C
Formation of amides
O
t
CH3 C
+ NH3
OH
+ SO2 + HCl
Cl
OH
O
CH3 C
+ H2O
NH2
Formation of acid anhydrides:
O
O
OH t , H2SO4(конц.) CH3 C
O + H2O
OH
CH3 C
CH3 C
O
O
(acetic anhydride)
CH3 C
С. Decarboxylation
140
ление
сплав
CH4 + Na2CO3
t
СН3СОСН3 + СаО + СО2
CH3COONa + NaOH
(СН3СОО)2Са
D. Radical reactions
CH3–CH2–COOH + Сl2 CH3–CHCl–COOH + HCl
Special properties of dicarboxylic acids
НООС–СООН
t
СО2 + НСООН
O
O
H2C C
H2C C
OH
OH
O
H2C C
t
O + H2O
H2C C
O
AMINO ACIDS AND PROTEINS
Aminoacids are the molecular blocks of the molecular structure of
the important and very complex class of compounds known as proteins.
Natural aminoacids are -aminoacids where amino- and carboxylic groups
occupy neighboring positions:
141
R CH COOH .
NH2
Some examples of aminoacids are presented below:
CH 2 COOH
Aminoacetic acid (glycine, Glu)
NH2
CH 3 CH COOH
NH2
H3C CH CH2
NH2
O
C
OH
HOOC CH 2 CH COOH
NH2
NH 2 CO CH 2 CH COOH
NH2
HOOC (CH 2 ) 2 CH COOH
NH2
NH 2 CO (CH 2 ) 2 CH COOH
NH2
-aminopropionic acid (alanine,
Ala)
3-aminobutiric acid (β-butiric acid)
Aspargic acid (Asp)
Aspargine (Asn)
Glutamic acid (Glt)
Glutamine (Gln)
HO CH 2 CH COOH
NH2
Serine (Ser)
HS CH 2 CH COOH
NH2
Cysteine (Cys)
CH 2 CH COOH
NH2
142
Phenylalanine (Phe)
HN
Histidine (His)
CH 2 CH COOH
NH2
N
Chemical properties
Aminoacids are amphoteric compounds which combine the
properties of weak organic acids and weak bases:
H2N–CH2–COOH + NaOH H2N–CH2–COONa + H2O
H2N–CH2–COOH + HCl Cl–H3N+–CH2–COOH
nature:
The solutions of amino acids are neutral because of its dipolar
H2N–CH2–COOH
H3N+–CH2–COO–
(Zwitterion)
Action at heat:
-aminoacids
O
H
N C
O
HO C
CH R
+
H2N
C OH
NH2
R CH
t
CH R
2H2O + R CH
C N
H
O
O
-aminoacids
R CH CH2 COOH
t
NH3 + R CH CH COOH
NH2
Other aminoacids
H2N(CH2)nCOOH
t
(CH2)n
C O
NH
143
Proteins are products of condensation of amino acids of different
nature:
OH
R1
R2
O
H2N CH C NH CH C
+ H2N CH C
H2N CH C
O
O
O
OH
R1
R2
OH
+ H2O
(dipeptide)
O
The
C NH
fragment is known as peptide (or amide) bond.
The dipeptide can further react with amino acids, forming long
polypeptide chains containing more than 40 amino acid residues, - proteins.
They are also called polypeptides. The molar masses of proteins are in the
range from 10,000 to several million au. The structure of natural proteins
are 20 -amino acids. Some of them may be synthesized by the body (nonessential amino acids), and the other only comes with food (essential amino
acids).
The order of amino acids in protein molecules is called the primary
structure of the protein. Due to intramolecular hydrogen bonds, protein
molecules fold into spirals, forming a secondary structure of the protein.
Helix, in turn, form the secondary coil (tertiary structure of the protein).
Spirals can be combined in pairs by intermolecular hydrogen bonds,
forming a quaternary structure.
Depending on the shape of macromolecules distinguish globular
(spherical) and fibrillar (filamentous) proteins. Under the influence of high
temperatures, strong acids and alkalis, salts, toxic metals, radiation,
quaternary, tertiary and secondary structure of proteins partially or
completely destroyes in the process of protein denaturation.
Qualitative reaction of peptides and proteins is the appearance
of red-purple color when added the slurry of cupric hydroxide in an alkali
to the protein solution (biuret reaction).
144
ESTERS, FATS AND OILS
Esters are products of the reaction of alcohols with carboxylic
acids (esterification)
O
H C
+ HO CH2 CH3
OH
O
реакция этерификации
H C
+ H2O
O CH2 CH3
ethylformiate
The major chemical property of esters is reaction of hydrolysis
(saponification) which is irreversible in alkaline media:
O
H C
+ H2O
O CH2 CH3
H+
O
H C
O
O
H C
+ HO CH2 CH3
OH
+ NaOH
O CH2 CH3
H C
+ HO CH2 CH3
ONa
Esters of carboxylic acids are divided into three groups:
1. Fruity esters. These are esters of lower monocarboxylic acids
and lower monovalent alcohols. They are liquids with a pleasant fruity and
floral scent:
Formula
Name
Scent
145
HCOOC2H5
ethylformiate
rome
HCOOCH2CH(CH3)2
isobutilformiate
strawberry
HCOOCH2C6H5
benzylformiate
jasmin
CH3COOC5H11
n-amylacetate
pear
CH3COOCH2CH2CH(CH3)2
isoamylacetate
banana
CH3COOC8H17
n-octylacetate
orange
C3H7COOC2H5
ethylbutirate
pinapple
Fruit esters are readily soluble in ethanol and diethyl ether, the
solubility in water decreases with increasing molar mass. They are used as
flavoring and fruit essences.
2. Waxes are esters of higher monobasic carboxylic acids and
higher monohydric alcohols. They are a colorless solids, which are used for
making candles, polishes and waxes floors, additives to soaps, lipsticks,
etc.
3. Fats and oils are esters of higher (fatty) monobasic carboxylic
acids and polyhydric alcohol - glycerol (triglycerides). Fats and oils are
natural products. If the carboxylic acid is saturated (stearic, palmitic acids),
the resulting ester is a fat (solid), and in the case of an unsaturated acid
(oleic, linoleic, linolenic), this is the oil (liquid material). Typically, solid
fats are of animal origin (beef and pork fat, butter), and liquid oils are
vegetable.
Fats are the feedstock for the manufacture of soap - sodium (solid
soap) or potassium (liquid soap) salts of higher carboxylic acids:
146
CH2 O CO C17H35
CH O CO C17H35 + 3NaOH
CH2 O CO C17H35
омыление
CH OH + 3C17H35COONa
CH2 OH
глицерин
тристеарат глицерина
(твердый жир)
glyceryl tristearate (fat)
CH2 OH
стеарат натрия
(твердое мыло)
glycerine sodium stearate (soap)
Soaps have detergent properties as can emulsify fats and oils, i.e.
convert them into fine droplets, which are wetted by water. Soaps can not
be used in hard water since the calcium and magnesium ions form insoluble
salts of fatty acids.
Hydrogenolysis of fats is the process of adding hydrogen to
unsaturated fatty acid residues that makes up the fat:
CH2 O CO C17H33
CH O CO C17H33 + 3H2
CH2 O CO C17H33
òðèî ëåàò ãëèöåðèí à
(ðàñòèòåëüí î å ì àñëî )
glyceryl trioleate (oil)
t, Ni
CH2 O CO C17H35
CH O CO C17H35
CH2 O CO C17H35
òðècòåàðàò ãëèöåðèí à
(ì àðãàðèí )
glyceryl tristearate (solid fat)
In this way, the conversion is carried out liquid fats (oils) in the
solid, which is used in the production of food margarine - a substitute for
butter.
Fats are important as a food product, and characterized by the
highest energy value. The technique fats are widely used for the
manufacture of varnishes and oil paints.
CARBOHYDRATES
147
Carbohydrates represent a class of organic compounds that
contain groups of natural compounds of plant and animal origin. Most of
the carbohydrate are of the general formula Cn(H2O)m. Carbohydrates
include monosaccharides - polyhydroxy aldehydes (aldoses) and
polyhydroxy ketones (ketoses), and their condensation products:
disaccharides, and polysaccharides. According to the number of carbon
atoms, monosaccharides are divided into tetroses (C4), pentose (C5), hexose
(C6), and heptose (C7). In nature, the most common are pentoses and
hexoses.
Monosacharides
Monosaccharides are the simpliest one-unit sugars. They contain
asymmetric carbon atoms, so that possess a large number of stereoisomers,
which are combined in pairs as optical antipodes - enantiomers. The
number of stereoisomers N = 2n, where n is the number of asymmetric
atoms in the molecule. For example, monosaccharides with four
asymmetric carbon atoms exist in the form of 16 stereoisomers
(enantiomers of 8 pairs of D- and L-series). Aldose D-series representing
the natural products are listed below as Fischer projections:
CHO
CHO
H
OH
HO
H
OH
H
CH2OH
D-erythrose
OH
CH2OH
D-threose
aldotetroses
148
H
CHO
CHO
H
OH
HO
H
OH
H
OH
HO
H
OH
H
OH
H
CH2OH
D-ribose
CHO
H
CHO
H
OH
HO
H
H
HO
H
OH
CH2OH
CH2OH
D-arabinose
D-xylose
aldopentoses
CHO
CHO
OH
CH2OH
D-lyxose
CHO
OH
HO
H
OH
H
OH
HO
H
OH
H
OH
H
OH
H
OH
H
OH
H
OH
H
OH
H
OH
CH2OH
D-altrose
CHO
H
OH
HO
H
OH
H
HO
H
H
OH
CH2OH
D-gulose
HO
H
H
H
H
H
CH2OH
D-allose
CHO
H
CHO
OH
HO
H
H
HO
H
CH2OH
D-glucose
CHO
H
CH2OH
D-mannose
CHO
OH
HO
H
OH
HO
H
HO
H
H
HO
H
HO
H
OH
H
OH
CH2OH
CH2OH
D-idose
D-galactose
aldohexoses
H
OH
CH2OH
D-talose
Diasteremerso are any combination of spatial isomers that are not a
pair of optical antipodes.
Pairs of diastereomers, differing in the configuration of one of
more asymmetric atoms, are called epimers. For example, the epimers are
149
D-ribose and D-arabinose, as they only differ in the disposition of the
substituents on the carbon atom in the 2nd position.
Ketoses are monosaccharides containing a carbonyl (ketone)
group. Some examples of ketoses are cited below:
CH2OH
CH2OH
O
HO
O
H
H
H
OH
HO
CH2OH
OH
H
CH2OH
rybulose
(product of photosynthesis in green
plants)
ketopentoses
xylulose
CH2OH
CH2OH
CH2OH
O
O
O
H
H
OH
H
H
OH
H
OH
HO
H
OH
H
OH
H
HO
CH2OH
fructose
CH2OH
psikose
ketohexoses
OH
H
OH
CH2OH
sorbose
Formation of cyclic forms of monosaccharides
The cyclization reaction is based on the ability of the alcohol and
the aldehyde groups of the molecules to react with one another. The carbon
chain containing sp3-carbons is bent, so a hydroxyl group at the fifth carbon
atom comes close to the aldehyde group and is able to react with it. The
formed during cyclization hydroxyl group at the 1st position can hold the
150
lower or upper position with respect to the plane of the ring, forming
respectively - and -forms of the sacharide. For example, the reaction of
cyclization of glucose can be presented as following:
H 1 O
C
H C OH
H
H 2
OH
HO
H
H
OH
H
OH
OH
H
O
H
O
H 5
H
CH2OH
CH2OH
CH2OH
CH2OH
6
CH2OH
H
H
H
OH
H
HO
6
CH2OH
OH
OH
4
H
H
OH
H
OH
OH
H
HO
OH
O
C
3
H
H
HO
4C
5C
O
H
OH
OH C
H
3
H
H
C2
O
C
1
O
H
H
OH
H
OH
H
H
OH
H
OH
OH
-D-glucose
-D-glucose
(glucopyranose)
(glucopyranose)
The cyclization of monosaccharides is a reversible process, so the
aldehyde group may be detected by a qualitative reaction (with Ag2O or
Cu(OH)2).
The hydroxyl group formed from the aldehyde group in the
cyclization is called a glycoside (acetal) group. The term "anomers" refers
to a pair of diastereomeric monosaccharide glycoside configuration
differing atom in cyclic form, for example α-D- anomer and β-D-glucose.
151
The cyclization of fructose can lead to the formation of five-or sixmembered rings:
CH2OH
C O
H
O
H
H
H
OH
OH
OH
H
OH
OH
H C OH
CH2OH
OH
CH2OH
HO C H
H C OH
CH2OH
H
Fructopyranose
H
O
HO
CH2OH
H
fructofuranose
Disaccharides
Disaccharides are products of condensation of monosaccharides
accompanied by an intermolecular dehydration. At formation of
disaccharides, the monosaccharides may be same or different. One of the
molecules participates in the reaction with its acetal OH group. The way of
adjustment of another monosaccharide may be different. For example, in
formation of sucrose (cane sugar), both OH fragments involving in the
dehydration are of the acetal character:
CH2OH
O
H
H
OH
H
OH
OH
H
OH
-D-glucose
152
H
CH2OH
H
+
H
O
HO
CH2OH
HO
OH
H
fructose
CH2OH
O
H
H
OH
H
CH2OH
H
H
H
O
HO
O
OH
H
OH
OH
+ H2O
CH2OH
H
sucros
e, С12Н22О11
(1-[
-D-fructofuranozyl]glucopyranozyde)
-D-
In this case, both the fragments of the disaccharide are in a stable
cyclic form, and neither aldehyde nor ketone group may be formed. Such
disaccharides are called non-reducing.
Tregalose is another example of non-reducing disaccharides. It
contains two glucose rests combined together through their acetal hydroxyl
groups:
Maltose is an example of a reducing disaccharide. Tho α-D
glucose units are combined in the way that one of the glucoside fragment is
not involved in condensation, and the cycle can open to produce an
aldehyde group which possess reducing properties:
153
Another example of reducing disaccharides are lactose and
cellobiose:
(lactose)
(cellobiose)
Polysaccharides
Polysaccharides are polycondensates of monosaccharides. Starch
and cellulose are polysaccharides formed by chains of glucose fragments:
nC6H12O6 (C6H10O5)n + nH2O.
Starch is a mixture of polysaccharides derived from -D-glucose. It
cans divided into two fractions: amylose (15 - 25%) and amylopectin (75 85%).
In amylose, -D-glucose residues are linked with hydroxyl groups
at the positions 1 and 4:
O
H
H
OH
CH2OH
CH2OH
CH2OH
H
O
H
H
OH
H
154
OH
O
H
H
OH
H
H
OH
H
H
O
O
O
H
H
H
OH
In amylopectin, the molecules of -D-glucose are linked through
the hydroxyl groups at positions 1, 4 and 6:
CH2OH
O
H
H
OH
H
H
O
H
CH2OH
O
H
H
OH
OH
H
CH2OH
CH2
O
H
H
OH
H
H
H
OH
H
H
H
O
O
O
H
O
H
H
OH
H
OH
OH
Cellulose consists of residues of -D-glucose linked through the
hydroxyl groups at positions 1 and 4:
H
OH
O
H
H
OH
OH
O
H
H
OH
H
HO
O
H
H
H
H
H
O
H
O
H
O
H
CH2OH
CH2OH
CH2OH
H
HO
Reactions of polysaccharides:
[C6H7O2(OH)3]n + 3nHO–NO2
cellulose
nitric acid
[C6H7O2(ONO2)3]n + 3nH2O
nitrocellulose (explosive)
[C6H7O2(OH)3]n + 3nHOОССН3 [C6H7O2(OОССН3)3]n + 3nH2O
cellulose
acetic acid
acetylcellulose (synthetic fibers)
155
SULPHUR IN ORGANIC COMPOUNDS
Organo-sulfur compounds are organic compounds that contain
sulfur. Due to the fact that sulfur is analog of oxygen, it is able to replace it
in the functional groups to form thiols (mercaptans), thioethers,
thioaldehydes, thioacid. The name of the organic sulfur compounds usually
appears prefix thio (thioalcohols thioaldehydes, monotio- and diotio
carboxylic acids). Thioderivatives have stronger acidic properties and a
very unpleasant odor; some are very toxic.
BIOLOGICALLY IMPORTANT HETEROCYCLIC
COMPOUNDS
Heterocyclic compounds (heterocycles) are organic compounds
containing rings whose composition comprises, together with the carbon
atoms of other elements (nitrogen, oxygen, sulfur). By biological
importance, nitrogen-containing heterocycles include five and six
membered cyclic compound having one or two nitrogen atoms, and their
condensed analogues are of a great significance.
Pyrrole and its derivatives
Pyrrole is a five- membered ring with one nitrogen atom. The lone
pair of electrons of the heteroatom (nitrogen) is involved in conjugation
with -electrons of the two double bonds, redistributing the electron
156
density. Thus, pyrrole readily undergoes electrophilic substitution on carbon atoms:
Due to the fact that the pair of electrons of nitrogen is shifted
towards the ring, the N-H bond is weakened and pyrrole exhibits acidic
properties:
+ 2K
2
+ H2
N
K
N
H
Pyrrole rings are part of the macrocyclic system – porphyrin. The
porphyrin in the blood forms a complex with ferrous iron, which is called
heme. Heme is in its turn connected with a coordination bond with globin
(a protein consisting of the amino acids of high molecular weight). So
constructed hemoglobin that is included in the composition of blood and
performs the function of the oxygen transport in the body.
CH CH2
H 3C
HC
N
H
H 3C
N
N
CH
CH
H
N
HC
HOOC CH2
CH
N
Fe
N
CH
N
HC
CH3
CH CH2
CH2
HOOC CH2
porphyrin
N
HC
CH2
CH3
hem
Porfirin ring is also a constituent of chlorophyll - the green pigment
in plants:
157
CH2 CH3
X
N
CH
N
Mg
N
CH
N
HC
H2C CH
C O
H 3C
HC
CH
COOCH3
H3C
CH2 CH2 COOC20H39
chlorophyll (Х = СН3 or СНО)
Condensation of the benzene ring to pyrrole molecule leads to the
formation benzopyrrol or indole. Among the derivatives of indole there is
an essential amino acid tryptophan, 3-(3-indolyl) -2-aminopropanoic acid:
CH2 CH COOH
NH2
N
H
N
H
indol
triptophan
Imidazole and its derivatives
Imidazole is a five-membered ring with two nitrogen atoms in
positions 1 and 3, one of the nitrogen atoms exhibits the basic properties,,
and the other is acidic, so imidazole is amphoteric:
N H+Cl-
N
+ HCl
N
H
158
N
H
N
N
+ NH3
+ NaNH2
N
H
N
Na
The aminoacid histidine is one of the most important natural
derivatives of imildazole. At elevated temperatures or under enzymatic
decomposition histidine is decarboxylated and converted to histamine:
N
N
H
CH2 CH COOH
t
N
N
H
NH2
histidine
CH2 CH2 NH2 + CO2
histamine
Pyridine and its derivatives
Pyridine is the most important six-membered heterocycle having
one nitrogen atom. Due to the fact that the pair of electrons of nitrogen
does not participate in conjugation with the -electron ring, pyridine
possesses basic properties, stronger than aniline, but weaker than aliphatic
amines:
+ HCl
N
N+ H Cl
Among pyridine derivatives there are nicotinic acid ( carboxypyridine), its amide (PP vitamin), pyridoxal, pyridoxol,
pyridoxamine (B6 vitamin), nicotine, etc.:
159
CONH2
COOH
N
N
Nicotinic acid
nicotinamide
CH2OH
CHO
HO
H3C
CH2OH
N
HO
CH2OH
H3C
pyridoxal
CH2NH2
HO
CH2OH
H3C
N
pyridoxol
N
pyridoxamin
N
N
CH3
nicotine
A condensed system of pyridine and benzene is quinoline. Pyridine
ring in it more aromatic, than benzene, so the total -bond belongs to a
greater extent to the pyridine ring. As a result, positions 5 and 8 of a
quinoline increase the activity of electrophilic substitution reactions:
5
6
NO2
4
10
3
+ 2HNO3
7
8
9
N
H2SO4
+ 2H2O
2
N
1
NO2
Quinoline moiety is a part of some drugs and quinoline alkaloids,
of which the most important is quinine (antimalarian):
160
CH CH2
CH2
CH2
HO CH
N
H3CO
N
Pyrimidine and purine bases
Pyrimidine is an example of the nitrogen-containing heterocyclic
compound having two nitrogen atoms. Pyrimidine cycles are included in the
nucleic acids associated with protein synthesis in cells. At the hydrolysis of
nucleic acids, three important pyrimidine derivatives (pyrimidine bases) are
formed. These are uracil (2,6-dihydroxypyrimidine), thymine (5-methyl-2,6dihydroxy-pyrimidine), and cytosine (6-amino-2-hydroxypyrimidine):
4
5
N
6
3
2
N
1
pyrimidine
H3C
N
N
NH2
OH
OH
OH
N
N
N
OH
N
OH
uracyl
thymine
cytosine
An important property of the pyrimidine bases is the keto-enol
(lactim-lactam) tautomerism:
161
O
OH
NH
N
N
N
H
OH
lactyme (enol)
O
lactame (ketone)
Along with the pyrimidine bases, the nucleic acids include purine pyrimidine condensed system and imidazole. Directly purine bases adenine
(6-aminopurine) and guanine (2-amino-6-hydroxypurine). participate in the
construction of nucleic acids
6
1
5
N
8
2
N
3
4
N9
H
purine
OH
NH2
7
N
N
N
N
N
H
adenine
N
N
H2N
N
N
H
guanine
Purine and pyrimidine bases play an important role in the
metabolism in the body. In conjunction with a monosaccharide (ribose or
deoxyribose), and phosphoric acid, they form the nucleotides which make up
the nucleic acids (DNA and RNA):
Fragment of a polymeric chain of
DNA
162
Purine derivatives are also included in the composition of alkaloids
(caffeine, theophylline and theobromine), toxins (e.g., saxitoxin) and other
related substances (uric acid):
O
H 3C
O
N
N
O
CH3
N
H3 C
N
O
CH3
O
N
N
N
N
H
CH3
N
HN
O
CH3
N
N
CH3
caffein
theophylline
theobromine
(coffee and tea
alkaloid)
(tea alkaloid)
(cacao alkaloid)
List of citated literature
1. Neil D. Jespersen. Chemistry: The Molecular Nature of Matter: 7th
Edition. Wiley, John & Sons, Incorporated, 2014.
163
2. Neil D. Jespersen. Student Solutions Manual to Accompany
Chemistry: The Molecular Nature of Matter: 7th Edition. Wiley, John
& Sons, Incorporated, 2014.
3. Rao C.N.R.
University General Chemistry. Macmillan India
Limited, 1997.
4. Bahl B.S., Bahl A. A textbook of Organic Chemistry. S Chand &
Company Ltd., 1997.
164
Oльга Владимировна Ковальчукова,
Насрин Намичемази,
Русул Алабада
Химия (конспект лекций).
Для студентов 1 курса медицинского факультета
специальности «Стоматология»
(на английском языке)
Зав. редакцией Т.О. Сергеева
Техн. редактор И.М.Любавская
Тематический план 2015 г., №
_______________________________________________________
Подписано в печать
Формат 60881/4. Ротапринтная печать.
Усл. печ. л. 10,25. Усл. кр.-отт. 10,25. Уч.-изд. № . Цена договорная
Издательство Российского университета дружбы народов
117923, ГСП-1, Москва, ул. Орджоникидзе,3
_________________________________________________________
Типография Российского университета дружбы народов
117923, ГСП-1, Москва, ул. Орджоникидзе,3
165