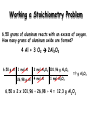* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 2 - Montville.net
Lewis acid catalysis wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Determination of equilibrium constants wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Isotopic labeling wikipedia , lookup
Chemical reaction wikipedia , lookup
Safety data sheet wikipedia , lookup
Atomic theory wikipedia , lookup
George S. Hammond wikipedia , lookup
Click chemistry wikipedia , lookup
Rate equation wikipedia , lookup
Electrolysis of water wikipedia , lookup
Transition state theory wikipedia , lookup
Gas chromatography–mass spectrometry wikipedia , lookup
Process chemistry wikipedia , lookup
Thermometric titration wikipedia , lookup
Vapor–liquid equilibrium wikipedia , lookup
Introduction Reaction stoichiometry involves the relationships between reactants and products in a chemical reaction. It is based on chemical equations similar to the ones studied in the last section. All reaction stoichiometry calculations start with a balanced equation. 3 C O + 4 H O C H + 5 O 2 2 3 8 2 You will need to be familiar with gram/mole relationships as studied earlier this year. Reaction stoichiometry is similar to cooking. (need correct ratios of ingredients) The mole enables chemists to move from the microscopic world of atoms and molecules to the real world of grams . Stoichiometry problems are classified between the information given in the problem and the information you are expected to find, the unknown. The given and the unknown may be expressed in grams or moles. The masses in the reaction are usually expressed in grams. Definition of mole: mole of a substance = grams of substance/MW of substance You will need to use: i. ii. iii. iv. molar ratios in a balanced equation. molar masses of reactants and products. balancing equations. conversions between grams and moles. Mole Ratios A mole ratio converts moles of one compound in a balanced chemical equation into moles of another compound. All stoichiometry problems use mole ratios. Example Reaction between magnesium and oxygen to form magnesium oxide. ( fireworks) 2 Mg(s) + O2(g) Mole Ratios: 2 : 1 2 MgO(s) (balanced) : 2 Stoichiometry (working with ratios) Ratios are found within a chemical equation. 2HCl + 1Ba(OH)2 2H2O + 1 BaCl2 coefficients give MOLAR RATIOS 2 moles of HCl react with 1 mole of Ba(OH)2 to form 2 moles of H2O and 1 mole of BaCl2 Practice Problems 1) N2 + 3 H2 ---> 2 NH3 Write the mole ratios for N2 to H2 and NH3 to H2. Review: Molar Mass A substance’s molar mass (molecular weight) is the mass in grams of one mole of the compound. CO2 = 44.01 grams per mole H2O = 18.02 grams per mole Ca(OH)2 = 74.10 grams per mole Review: Chemical Equations C2H5OH + 3O2 2CO2 + 3H2O reactants products 1 mole of ethanol reacts with 3 moles of oxygen to produce 2 moles of carbon dioxide and 3 moles of water Types of Stoichiometry Problems Problem Type 1: When you are given the amount of starting material in a reaction in moles and asked to calculate the amount of product in moles: amount of reactant in moles amount of product in moles Problem Type 2: When you are given the amount of starting material in moles and asked to calculate the mass of product in grams: amount of reactant in moles amount of product in moles mass of product in grams Problem Type 3: When you are given the mass of starting material in grams and asked to calculate the amount of product in moles. amount of reactant in grams amount of reactant in moles amount of product in moles Problem Type 4: 1. When you are given the mass of starting material in grams and asked to calculate the amount of product in grams. amount of reactant in grams amount of reactant in moles amount of product in moles amount of product in grams Problem Type 1: Given and unknown quantities are in moles Amount in moles of known substance Amount in moles of unknown substance How many moles of lithium hydroxide are required to react with 20 moles of CO2? C O + L i O H L i C O + H O 2 2 3 2 C O + 2 L i O H L i C O + H O b a l a n c e d e q u a t i o n 2 2 3 2 Given: amount of CO2 = 20 moles Unknown: amount of LiOH in moles Amount of CO2 in moles Amount of LiOH in moles mol CO2 x mol LiOH / mol CO2 = mol LiOH 20 mol CO2 x 2 mol LiOH / 1 mol CO2 = 40 mol LiOH mole ratio Problem Type 1 Mole – Mole Conversions When N2O5 is heated, it decomposes: 2N2O5(g) 4NO2(g) + O2(g) a. How many moles of NO2 can be produced from 4.3 moles of N2O5? 2N2O5(g) 4NO2(g) + O2(g) 4.3 mol ? mol Units match Mole – Mole Conversions When N2O5 is heated, it decomposes: 2N2O5(g) 4NO2(g) + O2(g) a. How many moles of NO2 can be produced from 4.3 moles of N2O5? 2N2O5(g) 4NO2(g) + O2(g) 4.3 mol ? mol Units match 4.3 mol N2O5 4mol NO2 2mol N 2O 5 = 8.6 moles NO2 b. How many moles of O2 can be produced from 4.3 moles of N2O5? 2N2O5(g) 4NO2(g) + O2(g) 4.3 mol 4.3 mol N2O5 1mol O 2 2mol N 2O 5 ? mol = 2.2 mole O2 Problem Type 2: Given amount is in moles and unknown quantity is in grams Amount in moles of known substance Amount in grams of unknown substance Problem Type 3: Given amount is in grams and unknown quantities are in moles Amount in grams of known substance Amount in moles of unknown substance Problem Type 2 mole ↔ gram In plants when carbon dioxide reacts with water it produces glucose and oxygen: 6CO2 + 6H2O(l) C6H12O6(s) + 6O2(g) How many grams of C6H12O6 is produced when 3.0 mol of water react with carbon dioxide? 6CO2 + 6H2O C6H12O6 + 6O2 3.0 mol 3.0 mol H2O 1mol C6H12O6 6mol H2O ? grams Units match 180.2g C6H12O6 = 90 g C6H12O6 1mol C6H12O6 Problem Type 2 mole ↔ gram In plants when carbon dioxide reacts with water it produces glucose and oxygen: 6CO2 + 6H2O(l) C6H12O6(s) + 6O2(g) How many grams of CO2 is needed to react with 3.0 mol of water? 6CO2 + 6H2O C6H12O6 + 6O2 ? grams 3.0 mol H2O 3.0 mol 6mol CO2 6mol H2O Units match 44g CO2 1mol CO2 = 132 g CO2 Problem Type 2 mole ↔ gram When magnesium burns in air, it combines with oxygen to form magnesium oxide according to the following equation: 2Mg + O2(g) 2MgO(s) How many grams of MgO is produced from 2.0 mol of magnesium? 2Mg + O2 2MgO 2.0 mol 2.0 mol Mg 2mol MgO 2mol Mg ? grams 40g MgO 1mol MgO Units match = 80 g MgO Problem Type 3 gram ↔ mole When N2O5 is heated, it decomposes: 2N2O5(g) 4NO2(g) + O2(g) a. How many moles of N2O5 were used if 210g of NO2 were produced? 2N2O5(g) 4NO2(g) + O2(g) ? moles 210g Units match 210 g NO2 1mol NO 2 46.0 g NO 2 2mol N 2O 5 4mol NO 2 = 2.28 moles N2O5 Problem Type 3 gram ↔ mole Nitric oxide is produced from the catalytic oxidation of ammonia NH3(g) + O2(g) NO(g) + H2O(g) a. How many moles of NO were produced from 824g of NH3? 4NH3(g) + 5O2 4NO(g) + 6H2O(g) 824g ? moles Units match 824 g NH3 1mol NH 3 17.04 g NH 3 4mol NO 4mol NH 3 = 48.4 moles NO Steps Involved in Solving Mass-Mass Stoichiometry Problems • Balance the chemical equation correctly • Using the molar mass of the given substance, convert the mass given to moles. • Determine the molar ratio. • Using the molar mass of the unknown substance, convert the moles just calculated to mass. Amount in grams of known substance mw Amount in moles of known molar substance ratio Amount in moles of unknown substance mw Amount in grams of unknown substance Problem Type 4 grams ↔ grams When N2O5 is heated, it decomposes: How many grams of N2O5 are needed to produce 75.0 grams of O2? 2N2O5(g) 4NO2(g) + O2(g) 75.0 g ? grams 75.0 g O2 mol O 2 32.0 g O 2 2mol N 2O 5 1mol O 2 108g N 2O 5 mol N 2O 5 = 506 grams N2O5 Stoichiometry Problem- Type 4 6.50 grams of aluminum reacts with an excess of oxygen. How many grams of aluminum oxide are formed. 1. Identify reactants and products and write the balanced equation. Al + O2 Al2O3 a. What are the reactants? b. What are the products? c. What are the balanced coefficients? Working a Stoichiometry Problem 6.50 grams of aluminum reacts with an excess of oxygen. How many grams of aluminum oxide are formed? 4 Al + 3 O2 2Al2O3 6.50 g Al 1 mol Al 2 mol Al2O3 101.96 g Al2O3 26.98 g Al 4 mol Al 1 mol Al2O3 = ? g Al2O3 6.50 x 2 x 101.96 ÷ 26.98 ÷ 4 = 12.3 g Al2O3 Acetylene gas (C2H2) is produced by adding water to calcium carbide: How many grams of acetylene are produced by adding water to 5.0 grams of CaC2? CaC2 + 2H2O C2H2 + Ca(OH)2 Balanced? 5.0 g 5.0 g CaC2 ? grams 1 mol CaC 2 1 mol C 2 H 2 26g C2H2 64.1 g CaC 2 1mol CaC 2 1 mol C2H2 = 2.0 grams C2H2 How many moles of CaC2 are needed to react completely with 49.0 g H2O: CaC2 + 2H20 C2H2(g) + Ca(OH)2 How many moles of CaC2 are needed to react 49.0 g of H20? CaC2 + 2H20 C2H2(g) + Ca(OH)2 ? moles 49.0g Units match 49 g H2O 1 mol H2O 18.0 g H2O 1 mol CaC2 2 mol H2O = 1.36 moles CaC2 Calculating the Percent Yield The predicted amount of product – which we have been doing with our stoichiometry problems has been for 100% yield, or the theoretical yield of a reaction. The theoretical yield is the maximum amount of product that can be produces in a given reaction. When chemical reactions take place, they are almost never 100% complete. A reaction may not go to 100% due to not all the reactants becoming involved, impurities in the reactants, competing side reactions, loss of product due to filtering, or just not getting it all out of the vessel. The actual yield is how much product can be collected – and measured. The percent yield is a ratio given by: Percent Yield = Actual Yield x 100 Theoretical Yield Calcium carbonate is decomposed by heating, as shown in the following equation: C a C O C a O + C O 3 2 1) What is the theoretical yield of this reaction if 24.8 g CaCO3 is heated to yield 13.1 g CaO? Handle like a typical stoichiometry problem: grams to grams Ignore the 13.1 g CaO to solve. This is the actual yield. 2) What is the percent yield? Chlorobenzene is used in the production of many different chemicals, such as aspirin, dyes and disinfectants. One method of preparing chlorobenzene is to react benzene C6H6, with chlorine according to the following equation: C6H6 + Cl2 C6H5Cl + HCl 1) When 36.8 g of C6H6 react with excess of Cl2, the actual yield of C6H5Cl is 38.8g? What is the theoretical yield? Handle like a typical stoichiometry problem: grams to grams 2) What is the percent yield? Standard Molar Volume Equal volumes of all gases at the same temperature and pressure contain the same number of molecules. - Amedeo Avogadro At STP (Standard Temperature and Pressure): 1 mole of a gas occupies 22.4 liters of volume Gas Stoichiometry If reactants and products are at the same conditions of temperature and pressure, then mole ratios of gases are also volume ratios. 3 H2(g) + N2(g) 2NH3(g) 3 moles H2 + 1 mole N2 2 moles NH3 3 liters H2 + 1 liter N2 2 liters NH3 Gas Stoichiometry #1 How many liters of ammonia gas can be produced when 12 liters of hydrogen gas react with an excess of nitrogen? 3 H2(g) + N2(g) 2NH3(g) 12 L H2 2 L NH3 3 L H2 = 8.0 L NH3 Gas Stoichiometry #2 How many liters of oxygen gas, at STP, can be collected from the complete decomposition of 50.0 grams of potassium chlorate? 2 KClO3(s) 2 KCl(s) + 3 O2(g) 50.0 g KClO3 1 mol KClO3 122.55 g KClO3 3 mol O2 22.4 L O2 2 mol KClO3 1 mol O2 = 13.7 L O2 Gas Stoichiometry #3 What mass of sulfur must be used to produce 12.61 L of gaseous sulfur dioxide at STP? S8(s) + 8 O2 8 SO2(g) 12.61 L SO2 1 mol SO2 1 mol S8 256 g S8 22.4 L SO2 8 mol SO2 1 mol S8 = 18.0 g S8