Heat Work
... When temperature changes, internal energy has changed – may happen through heat transfer or through mechanical work First law is a statement of conservation of energy Change in internal energy of system equals the difference between the heat added to the system and the work done by the ...
... When temperature changes, internal energy has changed – may happen through heat transfer or through mechanical work First law is a statement of conservation of energy Change in internal energy of system equals the difference between the heat added to the system and the work done by the ...
ph202_overhead_ch15
... • A measure of the disorder (or randomness) of a system • For a reversible the change in entropy is measured as the ratio of heat gained to temperature DS = (Q/T)R = Sfinal - Sinitial – When heat energy is gained by a system, entropy is gained by the system (and lost by the surrounding environment) ...
... • A measure of the disorder (or randomness) of a system • For a reversible the change in entropy is measured as the ratio of heat gained to temperature DS = (Q/T)R = Sfinal - Sinitial – When heat energy is gained by a system, entropy is gained by the system (and lost by the surrounding environment) ...
Heat and Thermodynamics
... Conduction is heat transfer by means of molecular agitation within a material without any motion of the material as a whole. If one end of a metal rod is at a higher temperature, then energy will be transferred down the rod toward the colder end because the higher speed particles will collide with t ...
... Conduction is heat transfer by means of molecular agitation within a material without any motion of the material as a whole. If one end of a metal rod is at a higher temperature, then energy will be transferred down the rod toward the colder end because the higher speed particles will collide with t ...
ENTROPY
... pressure, it is offered zero resistance. Hence, no work is one by or upon the gas. If this sounds unconvincing, consider ∫PextdV. Since the external pressure is constant, we take it out of the integral, and since the external pressure is zero, the whole term is zero by itself. w, then, too becomes z ...
... pressure, it is offered zero resistance. Hence, no work is one by or upon the gas. If this sounds unconvincing, consider ∫PextdV. Since the external pressure is constant, we take it out of the integral, and since the external pressure is zero, the whole term is zero by itself. w, then, too becomes z ...
Chapter 4
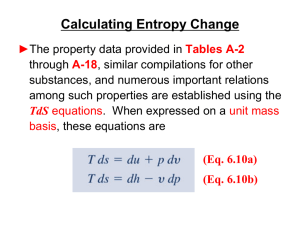
... ►The property data provided in Tables A-2 through A-18, similar compilations for other substances, and numerous important relations among such properties are established using the TdS equations. When expressed on a unit mass basis, these equations are (Eq. 6.10a) (Eq. 6.10b) ...
... ►The property data provided in Tables A-2 through A-18, similar compilations for other substances, and numerous important relations among such properties are established using the TdS equations. When expressed on a unit mass basis, these equations are (Eq. 6.10a) (Eq. 6.10b) ...
Thermodynamics
... A state variable describes the state of a system at time t, but it does not reveal how the system was put into that state. Examples of state variables: pressure, temperature, volume, number of moles, and internal energy. Thermal processes can change the state of a system. We assume that thermal proc ...
... A state variable describes the state of a system at time t, but it does not reveal how the system was put into that state. Examples of state variables: pressure, temperature, volume, number of moles, and internal energy. Thermal processes can change the state of a system. We assume that thermal proc ...
CARNOT CYCLE i) substance starts at with temperature T2
... Our next state function: ENTROPY (consequence of Second Law) There exists a function called entropy S, of the extensive variables of a system, defined for all equilibrium states, such that the values assumed by the extensive variables are those that maximize S (at equilibrium) From the viewpoint of ...
... Our next state function: ENTROPY (consequence of Second Law) There exists a function called entropy S, of the extensive variables of a system, defined for all equilibrium states, such that the values assumed by the extensive variables are those that maximize S (at equilibrium) From the viewpoint of ...
First law
... two regions become able to exchange matter or energy, tend to increase over time, approaching a maximum value when the jointly communicating system reaches thermodynamic equilibrium. In a simple manner, the second law states "energy systems have a tendency to increase their entropy rather than decre ...
... two regions become able to exchange matter or energy, tend to increase over time, approaching a maximum value when the jointly communicating system reaches thermodynamic equilibrium. In a simple manner, the second law states "energy systems have a tendency to increase their entropy rather than decre ...
Thermodynamics
... Positive when system gains heat Negative when system loses heat W = net work done by ...
... Positive when system gains heat Negative when system loses heat W = net work done by ...
ch06C-2013
... Isentropic Compressor and Pump Efficiencies ► Since the rate of entropy production cannot be negative, the only compressor exit states that can be attained in an adiabatic compression are those with s2 ≥ s1. This is shown on the Mollier diagram to the right. ► The state labeled 2s on the figure wou ...
... Isentropic Compressor and Pump Efficiencies ► Since the rate of entropy production cannot be negative, the only compressor exit states that can be attained in an adiabatic compression are those with s2 ≥ s1. This is shown on the Mollier diagram to the right. ► The state labeled 2s on the figure wou ...
Estimatin of Social Entropy - Research India Publications
... of social entropy. In thermodynamics, equilibrium occurs when is maximized, so that change in entropy (entropy production) is reduced to zero, as in Equation (3). Entropy is generally maximized as a result of all of the system’s energy being dissipated, so that the system is now in a state of rest o ...
... of social entropy. In thermodynamics, equilibrium occurs when is maximized, so that change in entropy (entropy production) is reduced to zero, as in Equation (3). Entropy is generally maximized as a result of all of the system’s energy being dissipated, so that the system is now in a state of rest o ...
Chapter 12: Thermodynamic Property Relations
... • The state postulate established that the state of a simple compressible system is completely specified by two independent, intensive properties. • Therefore, we should be able to calculate all the properties of a system such as internal energy, enthalpy, and entropy at any state once two independe ...
... • The state postulate established that the state of a simple compressible system is completely specified by two independent, intensive properties. • Therefore, we should be able to calculate all the properties of a system such as internal energy, enthalpy, and entropy at any state once two independe ...
Entropy in thermodynamics and information theory
There are close parallels between the mathematical expressions for the thermodynamic entropy, usually denoted by S, of a physical system in the statistical thermodynamics established by Ludwig Boltzmann and J. Willard Gibbs in the 1870s, and the information-theoretic entropy, usually expressed as H, of Claude Shannon and Ralph Hartley developed in the 1940s. Shannon, although not initially aware of this similarity, commented on it upon publicizing information theory in A Mathematical Theory of Communication.This article explores what links there are between the two concepts, and how far they can be regarded as connected.