A non-equilibrium quantum thermodynamics approach to
... of time, and the efficiency of motors are formulated in thermodynamic terms. In information theory, the definitions of information and entropy are explicitly related to this framework. More recently, it has been realised that thermodynamics can be used effectively to provide a new way of assessing a ...
... of time, and the efficiency of motors are formulated in thermodynamic terms. In information theory, the definitions of information and entropy are explicitly related to this framework. More recently, it has been realised that thermodynamics can be used effectively to provide a new way of assessing a ...
Example 1 First consider the case where there are no given
... The idea is that the function g should contain the same information as f . To get a feeling for this consider a function of one variable f (x) which is specified by the values over a set of points x. But we could also specify the function (up to some overall constant) by the values of the derivative ...
... The idea is that the function g should contain the same information as f . To get a feeling for this consider a function of one variable f (x) which is specified by the values over a set of points x. But we could also specify the function (up to some overall constant) by the values of the derivative ...
16 3.0 Chapter Contents 3.1 The Entropy and Internal Energy
... the chemical potentials. The concept of the chemical potential is again due to Gibbs (1948). The name reflects the fact that the internal energy, U, may be considered a potential for chemical work (of. w 8.4). Thus, we recognize the #kdNk (k = r + 1, ... )terms in Eq.(3.3)a as the mass action terms ...
... the chemical potentials. The concept of the chemical potential is again due to Gibbs (1948). The name reflects the fact that the internal energy, U, may be considered a potential for chemical work (of. w 8.4). Thus, we recognize the #kdNk (k = r + 1, ... )terms in Eq.(3.3)a as the mass action terms ...
Thermodynamics - StrikerPhysics
... remnant propellant gases under approximately 1 atm of pressure and 20°C. They display the warning “Do not dispose of this can in an incinerator”. What is the change in internal energy of such a gas if 500J of heat is added to it, raising the temperature to 2000°F? What is the final pressure of the g ...
... remnant propellant gases under approximately 1 atm of pressure and 20°C. They display the warning “Do not dispose of this can in an incinerator”. What is the change in internal energy of such a gas if 500J of heat is added to it, raising the temperature to 2000°F? What is the final pressure of the g ...
an energy flow analysis between mechanical systems based on the
... for which an invariant function E(x,i ) exists are considered. A wide class of mechanical resonators belong to this family of systems, since it is well known that the total energy is an invariant: ti(x,i)=o This property is valid provided that the system is isolated, i.e. it is left to itself, and t ...
... for which an invariant function E(x,i ) exists are considered. A wide class of mechanical resonators belong to this family of systems, since it is well known that the total energy is an invariant: ti(x,i)=o This property is valid provided that the system is isolated, i.e. it is left to itself, and t ...
Beginning Research on the Quantification of Spatial Order
... In 1906 Nernst formulated the Third Law of thermodynamics, which established that the entropy of a homogeneous solid or liquid body, at absolute zero temperature, is zero, which provides the basis for evaluation of absolute entropy, rather than just entropy change (Kondepudi & Prigogine 1998). Howev ...
... In 1906 Nernst formulated the Third Law of thermodynamics, which established that the entropy of a homogeneous solid or liquid body, at absolute zero temperature, is zero, which provides the basis for evaluation of absolute entropy, rather than just entropy change (Kondepudi & Prigogine 1998). Howev ...
The Laws of Thermodynamics
... information theory (Weaver and Shannon, 1949). In general, thermodynamics studies the transformation of energy of different types (e.g., thermal, solar, mechanical, chemical, electrical, etc., or work, heat, kinetic, potential, etc.) from one kind to another. Any transformation of energy must confor ...
... information theory (Weaver and Shannon, 1949). In general, thermodynamics studies the transformation of energy of different types (e.g., thermal, solar, mechanical, chemical, electrical, etc., or work, heat, kinetic, potential, etc.) from one kind to another. Any transformation of energy must confor ...
Basics of thermodynamics
... Helmholtz free energy minimum principle: The equilibrium value of any unconstrained internal parameter in a system in diathermal contact with a heat reservoir minimizes the Helmholtz free energy at constant temperature (the temperature of the reservoir) Enthalpy minimum principle: The equilibrium va ...
... Helmholtz free energy minimum principle: The equilibrium value of any unconstrained internal parameter in a system in diathermal contact with a heat reservoir minimizes the Helmholtz free energy at constant temperature (the temperature of the reservoir) Enthalpy minimum principle: The equilibrium va ...
Objectives Recognize that a system can absorb or release energy
... o A steady decrease in the car’s total mechanical energy occurs because of work being done against the _________________________ between the car’s axles and its bearings and between the car’s wheels and the coaster track. o If the internal energy for the roller coaster (the system) and the energy di ...
... o A steady decrease in the car’s total mechanical energy occurs because of work being done against the _________________________ between the car’s axles and its bearings and between the car’s wheels and the coaster track. o If the internal energy for the roller coaster (the system) and the energy di ...
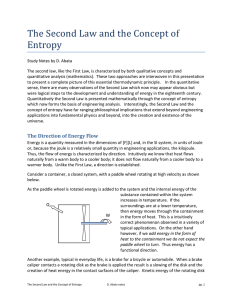
7. The Second Law and the Concept of Entropy
... described by the Third Law left for another discussion: The entropy of a substance is zero at absolute zero. Rudolf Clausius, a German born nineteenth century mathematician and physicist, in his work to further quantify the concept of heat mathematically derived entropy by dividing a quantity of hea ...
... described by the Third Law left for another discussion: The entropy of a substance is zero at absolute zero. Rudolf Clausius, a German born nineteenth century mathematician and physicist, in his work to further quantify the concept of heat mathematically derived entropy by dividing a quantity of hea ...
File - SPHS Devil Physics
... + Q = thermal energy absorbed by the gas (Qin) - Q = thermal energy lost by the gas (Qout) + W = work done by the gas (Wout) as it expands - W = work done on the gas (Win) to compress it + U = increase in internal energy/temperature - U = decrease in internal energy/temperature ...
... + Q = thermal energy absorbed by the gas (Qin) - Q = thermal energy lost by the gas (Qout) + W = work done by the gas (Wout) as it expands - W = work done on the gas (Win) to compress it + U = increase in internal energy/temperature - U = decrease in internal energy/temperature ...
ENTROPY
... In the second place, and more important, no on knows what entropy really is, so in a debate you will always have the advantage.”” Note that compound probabilities are multiplicative, uncertainties are additive and so is entropy. For equally-probable microstates totalising a number Ω, their probabili ...
... In the second place, and more important, no on knows what entropy really is, so in a debate you will always have the advantage.”” Note that compound probabilities are multiplicative, uncertainties are additive and so is entropy. For equally-probable microstates totalising a number Ω, their probabili ...
Isentropic Efficiency in Engineering Thermodynamics Introduction
... Examples are also given in the text for the isentropic efficiencies of nozzles and compressors, but they are all similar to the turbine example shown. Once you accept that a flowing fluid can have the properties of an equilibrium state, the rest follows. We see that the isentropic approximation is p ...
... Examples are also given in the text for the isentropic efficiencies of nozzles and compressors, but they are all similar to the turbine example shown. Once you accept that a flowing fluid can have the properties of an equilibrium state, the rest follows. We see that the isentropic approximation is p ...
Biogeochemical cycles and thermodynamics
... Since G = 0 at equilibrium, G < 0 implies disequilibria. Processes characterized by G > 0 violate the second law of thermodynamics and do not occur spontaneously. Gibbs free energy is expressed in Joules and is a measure of the maximum amount of energy available during transition from one state t ...
... Since G = 0 at equilibrium, G < 0 implies disequilibria. Processes characterized by G > 0 violate the second law of thermodynamics and do not occur spontaneously. Gibbs free energy is expressed in Joules and is a measure of the maximum amount of energy available during transition from one state t ...
The Second Law and the Concept of Entropy
... mathematical relationships1 and the absolute value of entropy is described by the Third Law left for another discussion: The entropy of a substance is zero at absolute zero. Rudolf Clausius, a German born nineteenth century mathematician and physicist, in his work to further quantify the concept of ...
... mathematical relationships1 and the absolute value of entropy is described by the Third Law left for another discussion: The entropy of a substance is zero at absolute zero. Rudolf Clausius, a German born nineteenth century mathematician and physicist, in his work to further quantify the concept of ...
ee10042808main.mov Example of Responding to an Unexpected
... Experiment session because students are vague about what entropy is. They also are vague about what it means to have entropy change as well as how to measure it or change it intentionally. Keeping it fixed, as they had in several of the partial derivatives considered during the first Name the Experi ...
... Experiment session because students are vague about what entropy is. They also are vague about what it means to have entropy change as well as how to measure it or change it intentionally. Keeping it fixed, as they had in several of the partial derivatives considered during the first Name the Experi ...
1 8. Entropy (Hiroshi Matsuoka) Why do we need entropy? There
... dU = TdS ! PdV + µ˜ dn , where µ˜ is called the chemical potential of the system. This extended form is useful when we deal with a quasi-static process in which the mole number of the system also changes because the system is connected with a particle reservoir that can exchange molecules or atoms w ...
... dU = TdS ! PdV + µ˜ dn , where µ˜ is called the chemical potential of the system. This extended form is useful when we deal with a quasi-static process in which the mole number of the system also changes because the system is connected with a particle reservoir that can exchange molecules or atoms w ...
S - BEHS Science
... Entropy changes for a reaction can be estimated in a manner analogous to that by which H is estimated: S° = nS°(products) - mS°(reactants) ...
... Entropy changes for a reaction can be estimated in a manner analogous to that by which H is estimated: S° = nS°(products) - mS°(reactants) ...
FE Review Common Pitfalls in Thermodynamics
... determine pressure as a function of volume so that the integral can be completed. 7. Integrals of PdV—Boundary work done by a working fluid within a system is given as the integral of PdV. Before one can successfully perform this integral, the process of the working fluid within the system must be k ...
... determine pressure as a function of volume so that the integral can be completed. 7. Integrals of PdV—Boundary work done by a working fluid within a system is given as the integral of PdV. Before one can successfully perform this integral, the process of the working fluid within the system must be k ...
Entropy in thermodynamics and information theory
There are close parallels between the mathematical expressions for the thermodynamic entropy, usually denoted by S, of a physical system in the statistical thermodynamics established by Ludwig Boltzmann and J. Willard Gibbs in the 1870s, and the information-theoretic entropy, usually expressed as H, of Claude Shannon and Ralph Hartley developed in the 1940s. Shannon, although not initially aware of this similarity, commented on it upon publicizing information theory in A Mathematical Theory of Communication.This article explores what links there are between the two concepts, and how far they can be regarded as connected.