Chapter 12: Thermodynamic Property Relations
... Some thermodynamic properties can be measured directly, but many others cannot. Therefore, it is necessary to develop some relations between these two groups so that the properties that cannot be measured directly can be evaluated. The derivations are based on the fact that properties are point fun ...
... Some thermodynamic properties can be measured directly, but many others cannot. Therefore, it is necessary to develop some relations between these two groups so that the properties that cannot be measured directly can be evaluated. The derivations are based on the fact that properties are point fun ...
Document
... Some thermodynamic properties can be measured directly, but many others cannot. Therefore, it is necessary to develop some relations between these two groups so that the properties that cannot be measured directly can be evaluated. The derivations are based on the fact that properties are point fun ...
... Some thermodynamic properties can be measured directly, but many others cannot. Therefore, it is necessary to develop some relations between these two groups so that the properties that cannot be measured directly can be evaluated. The derivations are based on the fact that properties are point fun ...
solutions
... Second Law efficiency is a measure of how much of the theoretical maximum (Carnot) you achieve, or in other words, a comparison of the system’s thermal efficiency to the maximum possible efficiency. The Second Law efficiency will always be between the Carnot and First Law efficiencies. ...
... Second Law efficiency is a measure of how much of the theoretical maximum (Carnot) you achieve, or in other words, a comparison of the system’s thermal efficiency to the maximum possible efficiency. The Second Law efficiency will always be between the Carnot and First Law efficiencies. ...
Test Thermodynamics Solutions
... or the measure of the quality of energy in natural processes and states if a process can happen in nature. 3rd Law of thermodynamics: it is impossible by any procedure, no matter how idealized, to reduce any system to absolute zero temperature in finite number of operations. List as many as you know ...
... or the measure of the quality of energy in natural processes and states if a process can happen in nature. 3rd Law of thermodynamics: it is impossible by any procedure, no matter how idealized, to reduce any system to absolute zero temperature in finite number of operations. List as many as you know ...
Period 6a Activity Solutions: Entropy
... b) Now watch the carts, without their outer covers, collide with the barrier. How can you explain the difference in the kinetic energy of the carts after they hit the barrier? More of the kinetic energy of motion of the elastic band cart is used to vibrate the washers. This kinetic energy goes into ...
... b) Now watch the carts, without their outer covers, collide with the barrier. How can you explain the difference in the kinetic energy of the carts after they hit the barrier? More of the kinetic energy of motion of the elastic band cart is used to vibrate the washers. This kinetic energy goes into ...
EQATION OF STATE IN FORM WHICH RELATES MOL FRACTION
... thermodynamic system consisted of an ideal gas. Indeed the ideal gas state equation connects well all the parameters in an ideal gas system. But if we try to solve the following problem: A thermodynamic system consists of two ideal gases A and B which are contained in some volume V (not given) at a ...
... thermodynamic system consisted of an ideal gas. Indeed the ideal gas state equation connects well all the parameters in an ideal gas system. But if we try to solve the following problem: A thermodynamic system consists of two ideal gases A and B which are contained in some volume V (not given) at a ...
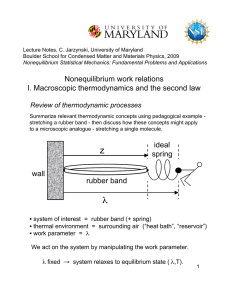
W - Boulder School for Condensed Matter and Materials Physics
... but keep in mind that the meaning of the quantities involved depends on how we define our system of interest. ...
... but keep in mind that the meaning of the quantities involved depends on how we define our system of interest. ...
Chemical Engineering Thermodynamics
... infinitesimally small, round spheres that occupy negligible volume and do not experience intermolecular attraction or repulsion. ...
... infinitesimally small, round spheres that occupy negligible volume and do not experience intermolecular attraction or repulsion. ...
Entropy. Temperature. Chemical Potential
... we view the closed system as composed of coupled subsystems (all macroscopic), then by definition these equilibria are obtained via an exchange of energy (U ), volume (V ) and particles (N ) among the subsystems. Equilibrium corresponds to the maximum value of the total entropy as a function of thes ...
... we view the closed system as composed of coupled subsystems (all macroscopic), then by definition these equilibria are obtained via an exchange of energy (U ), volume (V ) and particles (N ) among the subsystems. Equilibrium corresponds to the maximum value of the total entropy as a function of thes ...
[cond-mat.stat-mech] 29 Jul 1999 - Data Analysis and Modeling of
... In current usage, the terms “microscopically reversible” and “detailed balance” are often used interchangeably [26]. However, the original meaning of microscopic reversibility [27,28] is similar to Eq. (5). It relates the probability of a particular path to its reverse. This is distinct from the pri ...
... In current usage, the terms “microscopically reversible” and “detailed balance” are often used interchangeably [26]. However, the original meaning of microscopic reversibility [27,28] is similar to Eq. (5). It relates the probability of a particular path to its reverse. This is distinct from the pri ...
МІНІСТЕРСТВО ОХОРОНИ ЗДОРОВ`Я УКРАЇНИ ХАРКІВСЬКИЙ
... (oxidation with oxygen): heat effect of reaction is equal to sum of the standard enthalpies of combustion of reagents less the sum of standard enthalpies of combustion of products with account of stoichiometric coefficients. For living systems first law of thermodynamics can be formulated as follows ...
... (oxidation with oxygen): heat effect of reaction is equal to sum of the standard enthalpies of combustion of reagents less the sum of standard enthalpies of combustion of products with account of stoichiometric coefficients. For living systems first law of thermodynamics can be formulated as follows ...
Tutorial 1 / SS 2013
... The main constituents of every kind of FC are: Fuel input (e.g. gas of defined pressure) to maintain constant performance (current), metal electrodes to collect the current, a supported catalyst to drive the redox reactions (the reactions take place at the interface of the supported catalyst and the ...
... The main constituents of every kind of FC are: Fuel input (e.g. gas of defined pressure) to maintain constant performance (current), metal electrodes to collect the current, a supported catalyst to drive the redox reactions (the reactions take place at the interface of the supported catalyst and the ...
The Laws of Thermodinamics
... more probable than an orderly one if the laws of nature are allowed to act without interference ...
... more probable than an orderly one if the laws of nature are allowed to act without interference ...
Chapter 1 Thermodynamics
... Problem: Prove that the Kelvin and the Clausius statements of the Second Law are equivalent. Solution: Assume that Kelvin statement is false ∆ Extract Q of heat from a reservoir at temperature T2 and convert it entirely to work W = Q. Then convert this work back into heat Q = W and transfer it to re ...
... Problem: Prove that the Kelvin and the Clausius statements of the Second Law are equivalent. Solution: Assume that Kelvin statement is false ∆ Extract Q of heat from a reservoir at temperature T2 and convert it entirely to work W = Q. Then convert this work back into heat Q = W and transfer it to re ...
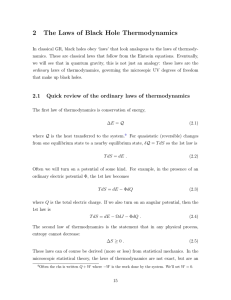
2 The Laws of Black Hole Thermodynamics
... This is conserved along the path of the particle (which is not a geodesic, since it feels an electromagnetic force). For a charged particle on the Reissner-Nordstrom black hole, find ✏ in terms of f (r) and the components of pµ . (b) Assume Q > 0. For one sign of q, the energy ✏ can be negative. Whi ...
... This is conserved along the path of the particle (which is not a geodesic, since it feels an electromagnetic force). For a charged particle on the Reissner-Nordstrom black hole, find ✏ in terms of f (r) and the components of pµ . (b) Assume Q > 0. For one sign of q, the energy ✏ can be negative. Whi ...
ONSAGER`S VARIATIONAL PRINCIPLE AND ITS APPLICATIONS
... dU = T dS − ΠdV. We see that the change of internal energy consists of two parts. The term T dS represents the change in U when the external parameters are kept constant (dV = 0). This is what we mean by heat. Thus DQ = T dS is the quantity of heat added to the system in a reversible process. The sy ...
... dU = T dS − ΠdV. We see that the change of internal energy consists of two parts. The term T dS represents the change in U when the external parameters are kept constant (dV = 0). This is what we mean by heat. Thus DQ = T dS is the quantity of heat added to the system in a reversible process. The sy ...
THERMODYNAMICS
... In this chapter you’ll learn that reactions not only change in enthalpy, but also in another important thermodynamic quantity, entropy (related to randomness) ...
... In this chapter you’ll learn that reactions not only change in enthalpy, but also in another important thermodynamic quantity, entropy (related to randomness) ...
Unit II - Chemical Thermodynamics
... Consider two heat engines, one reversible and other irreversible. We assume the both the engines absorb same amount of heat QH from the heat source having the temperature of TH. Both the engines reject the heat QL to a heat sink at a temperature TL . Applying the first law of thermodynamics to both ...
... Consider two heat engines, one reversible and other irreversible. We assume the both the engines absorb same amount of heat QH from the heat source having the temperature of TH. Both the engines reject the heat QL to a heat sink at a temperature TL . Applying the first law of thermodynamics to both ...
Questions - TTU Physics
... oscillator. So, the quantum mechanics of it is approximately the same as it is for the quantum mechanical harmonic oscillator. Using the approximate quantum mechanical expression for the energy E of the same pendulum as in part b, estimate the quantum number n. Compare this result to the results of ...
... oscillator. So, the quantum mechanics of it is approximately the same as it is for the quantum mechanical harmonic oscillator. Using the approximate quantum mechanical expression for the energy E of the same pendulum as in part b, estimate the quantum number n. Compare this result to the results of ...
Exam 1 - BYU Physics
... isolated from the rest of the universe. Which of the following is true? a. The entropy of the gas will increase. The entropy of the reservoir will increase. b. The entropy of the gas will increase. The entropy of the reservoir will decrease. c. The entropy of the gas will increase. The entropy of th ...
... isolated from the rest of the universe. Which of the following is true? a. The entropy of the gas will increase. The entropy of the reservoir will increase. b. The entropy of the gas will increase. The entropy of the reservoir will decrease. c. The entropy of the gas will increase. The entropy of th ...
The Second Law of Thermodynamics, Preview of
... a thermodynamic state (p1 , V1 , T1 ). We want to double the volume of the gas. One way to do this is through an adiabatic expansion governed by the adiabatic ideal gas law p1 V1γ = p2 V2γ If the gas volume is kept insulated from any heat exchange with the outside world, and the gas gradually expand ...
... a thermodynamic state (p1 , V1 , T1 ). We want to double the volume of the gas. One way to do this is through an adiabatic expansion governed by the adiabatic ideal gas law p1 V1γ = p2 V2γ If the gas volume is kept insulated from any heat exchange with the outside world, and the gas gradually expand ...
Thermodynamics: Heat and Work
... • In reality energy is transferred as both heat and work to some extent. • However, in most cases one type of energy transfer dwarfs the other. • Therefore, we can use ideal processes to approximate ...
... • In reality energy is transferred as both heat and work to some extent. • However, in most cases one type of energy transfer dwarfs the other. • Therefore, we can use ideal processes to approximate ...
New Microsoft Office Word Document
... Thermodynamic Equilibrium:- A system in which all macroscopic properties do not undergo any change with time Thermal Equilibrium:- If there is no heat exchange from one portion of the system to the another portion of the system, the system is said to be in Thermal Equilibrium Mechanical Equilibrium: ...
... Thermodynamic Equilibrium:- A system in which all macroscopic properties do not undergo any change with time Thermal Equilibrium:- If there is no heat exchange from one portion of the system to the another portion of the system, the system is said to be in Thermal Equilibrium Mechanical Equilibrium: ...
Entropy in thermodynamics and information theory
There are close parallels between the mathematical expressions for the thermodynamic entropy, usually denoted by S, of a physical system in the statistical thermodynamics established by Ludwig Boltzmann and J. Willard Gibbs in the 1870s, and the information-theoretic entropy, usually expressed as H, of Claude Shannon and Ralph Hartley developed in the 1940s. Shannon, although not initially aware of this similarity, commented on it upon publicizing information theory in A Mathematical Theory of Communication.This article explores what links there are between the two concepts, and how far they can be regarded as connected.







![[cond-mat.stat-mech] 29 Jul 1999 - Data Analysis and Modeling of](http://s1.studyres.com/store/data/004609137_1-3d6203405239cf93abc08201b80fbc47-300x300.png)