Lecture #6 09/14/04
... Formally, an ensemble is virtual construct of many copies of a system of interest. Each member of an ensemble has some mechanic or thermodynamic variables fixed, but all states corresponding to these fixed variables all allowed. Each state is represented equally in an ensemble; or alternatively an i ...
... Formally, an ensemble is virtual construct of many copies of a system of interest. Each member of an ensemble has some mechanic or thermodynamic variables fixed, but all states corresponding to these fixed variables all allowed. Each state is represented equally in an ensemble; or alternatively an i ...
2 nd Law of Thermodynamics
... Microscopic Statistical Physics Fundamental Statistical Physics Concept # of accessible microstates for a macroscopic system with huge particle number N + huge number f degrees of freedom. ...
... Microscopic Statistical Physics Fundamental Statistical Physics Concept # of accessible microstates for a macroscopic system with huge particle number N + huge number f degrees of freedom. ...
chapter12_PC
... more probable than an orderly one if the laws of nature are allowed to act without interference ...
... more probable than an orderly one if the laws of nature are allowed to act without interference ...
The First, Second, and Third Law of Thermodynamics (ThLaws05.tex)
... The laws of thermodynamics apply to well-de…ned systems. First we will discuss a quite general form of the …rst and second law. I.e. we consider a system which is inhomogeneous, we allow mass transfer across the boundaries (open system), and we allow the boundaries to move. Fig.1 is a general repres ...
... The laws of thermodynamics apply to well-de…ned systems. First we will discuss a quite general form of the …rst and second law. I.e. we consider a system which is inhomogeneous, we allow mass transfer across the boundaries (open system), and we allow the boundaries to move. Fig.1 is a general repres ...
Lecture_1 - Biman Bagchi
... of non-interacting atoms, such a classical ideal gas, does not show any phase transition. For example, why do we have to supercool most liquids below freezing point to grow crystals? Why liquid sodium does freeze to a bcc crystalline phase instead of an fcc phase, while iron freezes to an fcc phase? ...
... of non-interacting atoms, such a classical ideal gas, does not show any phase transition. For example, why do we have to supercool most liquids below freezing point to grow crystals? Why liquid sodium does freeze to a bcc crystalline phase instead of an fcc phase, while iron freezes to an fcc phase? ...
Lecture VIII_IX
... temperature and expansion L is given by f(T,L) = aT(L-L0) where a and L0 are constants. • (a)Use Maxwell relations to determine the entropy and enthalpy at constant T and p. • (b) If you adiabatically stretch a rubber band by small amount, its temperature increases but volume does not change. Derive ...
... temperature and expansion L is given by f(T,L) = aT(L-L0) where a and L0 are constants. • (a)Use Maxwell relations to determine the entropy and enthalpy at constant T and p. • (b) If you adiabatically stretch a rubber band by small amount, its temperature increases but volume does not change. Derive ...
unit ii chemical thermodynamics
... Second law Entropy - entropy change for an ideal gas, reversible and irreversible processes ,entropy of phase transitions. Clausius inequality. Free energy and work function: Helmholtz and Gibbs free energy functions Criteria of spontaneity Gibbs-Helmholtz equation Clausius-Clapeyron ...
... Second law Entropy - entropy change for an ideal gas, reversible and irreversible processes ,entropy of phase transitions. Clausius inequality. Free energy and work function: Helmholtz and Gibbs free energy functions Criteria of spontaneity Gibbs-Helmholtz equation Clausius-Clapeyron ...
Bioengineering Thermodynamics - PORTO
... approach in order to improve this science by analysing the biosystems also from a thermal point of view. Indeed, the fundamental role of thermodynamics in biology can be pointed out in some fundamental results in literature. Wilson, et al. [8] developed a thermodynamic relationships in mitochondrial ...
... approach in order to improve this science by analysing the biosystems also from a thermal point of view. Indeed, the fundamental role of thermodynamics in biology can be pointed out in some fundamental results in literature. Wilson, et al. [8] developed a thermodynamic relationships in mitochondrial ...
Chapter 2 Classical Thermodynamics: The Second Law 2.1 Heat
... Also, from 1st law, dE = d̄Q + d̄W = d̄Qrev + d̄W rev = d̄Qirrev + d̄W irrev and, by definition of reversibility, for a given change of the system (e.g., given dV for a gas), d̄W irrev > d̄W rev (to overcome friction, etc.), we have d̄Qirrev < d̄Qrev , or d̄Q ≤ T dS ...
... Also, from 1st law, dE = d̄Q + d̄W = d̄Qrev + d̄W rev = d̄Qirrev + d̄W irrev and, by definition of reversibility, for a given change of the system (e.g., given dV for a gas), d̄W irrev > d̄W rev (to overcome friction, etc.), we have d̄Qirrev < d̄Qrev , or d̄Q ≤ T dS ...
Statistical mechanics
... Statistical mechanics or statistical thermodynamics[1] is a branch of physics that applies probability theory, which contains mathematical tools for dealing with large populations, to the study of the thermodynamic behavior of systems composed of a large number of particles. Statistical mechanics pr ...
... Statistical mechanics or statistical thermodynamics[1] is a branch of physics that applies probability theory, which contains mathematical tools for dealing with large populations, to the study of the thermodynamic behavior of systems composed of a large number of particles. Statistical mechanics pr ...
Entanglement Entropies in the Ground States of Helium
... since it is directly calculable from the integral representation and does not require diagonalization of the RDM. Comparing the dependence on Z of the linear and vN entropies, we noted that from Z = 2 to Z = 5 they are almost linearly related. This is demonstrated in Fig. 1, where the vN entropy is ...
... since it is directly calculable from the integral representation and does not require diagonalization of the RDM. Comparing the dependence on Z of the linear and vN entropies, we noted that from Z = 2 to Z = 5 they are almost linearly related. This is demonstrated in Fig. 1, where the vN entropy is ...
Thermodynamics
... 2.00 x 105 Pa from a volume of 2.00 m3 to a volume of 0.500 m3. What is the work done on the gas. If the temperature initially was 40˚ C what is the final temperature of the gas? ...
... 2.00 x 105 Pa from a volume of 2.00 m3 to a volume of 0.500 m3. What is the work done on the gas. If the temperature initially was 40˚ C what is the final temperature of the gas? ...
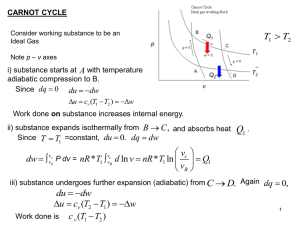
The Laws of Thermodynamics
... The working substance (air in this case) produces a net amount of work during each cycle. This is clear from the p-V diagram. The work done by the air is ∫pdV which is the area of the cycle on the p-V diagram. The direction in which the cycle is followed determines whether work is done by the air or ...
... The working substance (air in this case) produces a net amount of work during each cycle. This is clear from the p-V diagram. The work done by the air is ∫pdV which is the area of the cycle on the p-V diagram. The direction in which the cycle is followed determines whether work is done by the air or ...
Thermodynamics and the aims of statistical mechanics
... and at a particular rate. We call this a tangent vector, and it clearly defines the velocities q̇i of all the particles. From Newton’s second law (“F = ma”), together with the assumption that all forces will be a function only of positions and velocities, we know that a specification of all N positi ...
... and at a particular rate. We call this a tangent vector, and it clearly defines the velocities q̇i of all the particles. From Newton’s second law (“F = ma”), together with the assumption that all forces will be a function only of positions and velocities, we know that a specification of all N positi ...
Absolute Rate Theory
... In this equation, k is the bimolecular rate constant for conversion of A and B to P and k' is the unimolecular rate constant for decomposition of the activated complex (AB)‡ to form P. Now, the Eyring approach assumes that we can assume a thermodynamic quasi-equilibrium to exist between A, B and (AB ...
... In this equation, k is the bimolecular rate constant for conversion of A and B to P and k' is the unimolecular rate constant for decomposition of the activated complex (AB)‡ to form P. Now, the Eyring approach assumes that we can assume a thermodynamic quasi-equilibrium to exist between A, B and (AB ...
11 Thermodynamics and Thermochemistry
... constant pressure. When a reaction is allowed to take place in an open container a quantity of heat proportional to the quantity of matter present, will be released or absorbed. This flow of heat is the enthalpy change, ∆H. The units for ∆H are kJ (or kJ/mol). «Reactions that release heat are termed ...
... constant pressure. When a reaction is allowed to take place in an open container a quantity of heat proportional to the quantity of matter present, will be released or absorbed. This flow of heat is the enthalpy change, ∆H. The units for ∆H are kJ (or kJ/mol). «Reactions that release heat are termed ...
Dissipation and the so-called entropy production
... towards a (transient) nonequilibrium “steady” state, averages of smooth phases functions in those transient dissipative states can be approximated as NESS averages. In our system the application of infinite checkerboard boundary conditions means that space is translationally homogeneous but orienta ...
... towards a (transient) nonequilibrium “steady” state, averages of smooth phases functions in those transient dissipative states can be approximated as NESS averages. In our system the application of infinite checkerboard boundary conditions means that space is translationally homogeneous but orienta ...
ch06A-2013
... Entropy Rate Balance for Control Volumes Comment: The value of the entropy production for a single component such as the throttling valve considered here often does not have much significance by itself. The significance of the entropy production of any component is normally determined through compa ...
... Entropy Rate Balance for Control Volumes Comment: The value of the entropy production for a single component such as the throttling valve considered here often does not have much significance by itself. The significance of the entropy production of any component is normally determined through compa ...
Engineering Building Room 2303 Mail Code Phone: 818-677
... per-unit mole basis, where the number of moles, n = m / M. M is the molecular weight. We can use the symbol v = V / n = v / M for the volume per unit mole. Similarly, u = U / n = u / M, h = H / n = h / M, s = S / n = s / M, a = A / n = a / M, and ...
... per-unit mole basis, where the number of moles, n = m / M. M is the molecular weight. We can use the symbol v = V / n = v / M for the volume per unit mole. Similarly, u = U / n = u / M, h = H / n = h / M, s = S / n = s / M, a = A / n = a / M, and ...
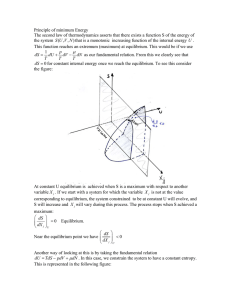
Principle of minimum Energy The second law of thermodynamics
... TdStotal = − dFsystem + δ Wsystem (irrev) ≥ 0 or if δ Wsystem = 0 then dFsystem ≤ 0 dFsystem = d (U − TS ) = dU − TdS ≤ 0 Therefore F reaches a minimum at equilibrium A system that is isothermal with a bath, and that can only exchange heat but NO WORK will try to minimize its free energy F. Any isot ...
... TdStotal = − dFsystem + δ Wsystem (irrev) ≥ 0 or if δ Wsystem = 0 then dFsystem ≤ 0 dFsystem = d (U − TS ) = dU − TdS ≤ 0 Therefore F reaches a minimum at equilibrium A system that is isothermal with a bath, and that can only exchange heat but NO WORK will try to minimize its free energy F. Any isot ...
PPT
... Our next state function: ENTROPY (consequence of Second Law) There exists a function called entropy S, of the extensive variables of a system, defined for all equilibrium states, such that the values assumed by the extensive variables are those that maximize S (at equilibrium) From the viewpoint of ...
... Our next state function: ENTROPY (consequence of Second Law) There exists a function called entropy S, of the extensive variables of a system, defined for all equilibrium states, such that the values assumed by the extensive variables are those that maximize S (at equilibrium) From the viewpoint of ...
Entropy in thermodynamics and information theory
There are close parallels between the mathematical expressions for the thermodynamic entropy, usually denoted by S, of a physical system in the statistical thermodynamics established by Ludwig Boltzmann and J. Willard Gibbs in the 1870s, and the information-theoretic entropy, usually expressed as H, of Claude Shannon and Ralph Hartley developed in the 1940s. Shannon, although not initially aware of this similarity, commented on it upon publicizing information theory in A Mathematical Theory of Communication.This article explores what links there are between the two concepts, and how far they can be regarded as connected.