NATURE OF ENTROPY OF MIXING
... The heat transfer from a cool body to a warmer one does not break the second law of thermodynamics since here the work is spent off. The operation of refrigerators is based on this principle, however in this case to transfer heat an intermediate link - a coolant - working body is needed which with t ...
... The heat transfer from a cool body to a warmer one does not break the second law of thermodynamics since here the work is spent off. The operation of refrigerators is based on this principle, however in this case to transfer heat an intermediate link - a coolant - working body is needed which with t ...
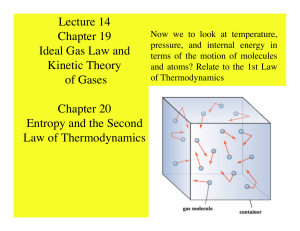
Lecture 14 Chapter 19 Ideal Gas Law and Kinetic Theory of Gases
... also called the molar mass. 1 mole of air contains 29 gms Then the number of moles in 1.2 kg is ...
... also called the molar mass. 1 mole of air contains 29 gms Then the number of moles in 1.2 kg is ...
Thermodynamics
... As time passes in an isolated system, internal differences in the system tend to even out (e.g., pressures and temperatures tend to equalize, as do density differences). A system in which all equalizing processes have gone practically to completion, is considered to be in a state of thermodynamic eq ...
... As time passes in an isolated system, internal differences in the system tend to even out (e.g., pressures and temperatures tend to equalize, as do density differences). A system in which all equalizing processes have gone practically to completion, is considered to be in a state of thermodynamic eq ...
Physics 201 - University of Virginia
... QL of all these heat engines contributes to warming of the atmosphere and water. This is an inevitable consequence of the second law of thermodynamics. ...
... QL of all these heat engines contributes to warming of the atmosphere and water. This is an inevitable consequence of the second law of thermodynamics. ...
Vijay Ramani, J. M. Fenton Thermodynamics of Fuel Cells
... fundamental to the second law of thermodynamics. A system is said to undergo a reversible change if it remains in equilibrium as it passes from its initial state to its final state. A reversible process is a reversible change in which the system remains in equilibrium with its environment. The visua ...
... fundamental to the second law of thermodynamics. A system is said to undergo a reversible change if it remains in equilibrium as it passes from its initial state to its final state. A reversible process is a reversible change in which the system remains in equilibrium with its environment. The visua ...
BOLTZMANN`S ENTROPY AND TIME`S ARROW
... problem is to identify the time order in which the sequence of snapshots was taken. The obvious answer, based on experience, is that time increases from figure 2a to 2b to 2c to 2d—any other order is clearly absurd. Now it would be very simple and nice if this answer followed directly from the micro ...
... problem is to identify the time order in which the sequence of snapshots was taken. The obvious answer, based on experience, is that time increases from figure 2a to 2b to 2c to 2d—any other order is clearly absurd. Now it would be very simple and nice if this answer followed directly from the micro ...
boltzmann`s entropy and time`s arrow
... problem is to identify the time order in which the sequence of snapshots was taken. The obvious answer, based on experience, is that time increases from figure 2a to 2b to 2c to 2d—any other order is clearly absurd. Now it would be very simple and nice if this answer followed directly from the micro ...
... problem is to identify the time order in which the sequence of snapshots was taken. The obvious answer, based on experience, is that time increases from figure 2a to 2b to 2c to 2d—any other order is clearly absurd. Now it would be very simple and nice if this answer followed directly from the micro ...
Carnot - UniMAP Portal
... • Chemical thermodynamics studies PV work, which occurs when the volume of a fluid changes. PV work is represented by the following differential equation: • where: – W = work done on the system – P = external pressure – V = volume ...
... • Chemical thermodynamics studies PV work, which occurs when the volume of a fluid changes. PV work is represented by the following differential equation: • where: – W = work done on the system – P = external pressure – V = volume ...
Document
... The first law of thermodynamics states the equivalence of heat and work and reaffirms the principle of conservation of energy. The second law states that heat does not of itself pass from a cooler to a hotter body. Another, equivalent, formulation of the second law is that the entropy of a closed sy ...
... The first law of thermodynamics states the equivalence of heat and work and reaffirms the principle of conservation of energy. The second law states that heat does not of itself pass from a cooler to a hotter body. Another, equivalent, formulation of the second law is that the entropy of a closed sy ...
Course Home - Haldia Institute of Technology
... Different numerical problems Surface tension (cohesion, adhesion). ST on liquid droplets, soap bubble, liquid jet. Numerical problems. Capillarity: capillary rise and depression. Numerical problems. Fluid statics: pressure, Pascal’s law. Basic equation of fluid statics. Absolute, atmospheric, gauge ...
... Different numerical problems Surface tension (cohesion, adhesion). ST on liquid droplets, soap bubble, liquid jet. Numerical problems. Capillarity: capillary rise and depression. Numerical problems. Fluid statics: pressure, Pascal’s law. Basic equation of fluid statics. Absolute, atmospheric, gauge ...
Thermodynamics
... from your coffee. While some processes appear to be completely reversible, in practice, none actually are. Entropy, therefore, provides us with an arrow of time: forward is the direction of increasing entropy. ...
... from your coffee. While some processes appear to be completely reversible, in practice, none actually are. Entropy, therefore, provides us with an arrow of time: forward is the direction of increasing entropy. ...
Chemical Bonds in Biochemistry - Biochemistry
... 1.3.3. Entropy and the Laws of Thermodynamics The highly structured, organized nature of living organisms is apparent and astonishing. This organization extends from the organismal through the cellular to the molecular level. Indeed, biological processes can seem magical in that the well-ordered str ...
... 1.3.3. Entropy and the Laws of Thermodynamics The highly structured, organized nature of living organisms is apparent and astonishing. This organization extends from the organismal through the cellular to the molecular level. Indeed, biological processes can seem magical in that the well-ordered str ...
THERMODYNAMICS
... The entropy of 1 mole of vapor is calculated using the entropy of 1 mole of liquid (161 J/K) to which the entropy change resulting from the heat absorption (141.9 J/K) is added: Entropy of Vapor = 161 J/K + 141.9 J/K = 303 J/K 3. Consider the combustion of propane gas: C3H8 (g) + 5 O2 (g) → 3 CO2 (g ...
... The entropy of 1 mole of vapor is calculated using the entropy of 1 mole of liquid (161 J/K) to which the entropy change resulting from the heat absorption (141.9 J/K) is added: Entropy of Vapor = 161 J/K + 141.9 J/K = 303 J/K 3. Consider the combustion of propane gas: C3H8 (g) + 5 O2 (g) → 3 CO2 (g ...
15.3 The First Law of Thermodynamics
... Thermodynamics is the branch of physics that is built upon the fundamental laws that heat and work obey. ...
... Thermodynamics is the branch of physics that is built upon the fundamental laws that heat and work obey. ...
THERMODYNAMIC REVIEW PROBLEMS: ME 435 Fall 2004
... and what some sources of irreversibility are 4) Second Law limits on the performance of power cycles, heat pumps, and refrigerators 5) the temperature scale and its effect on calculating the second law limits for cycles 6) the Carnot cycle (power cycle or heat pump or refrigerator): what it is (the ...
... and what some sources of irreversibility are 4) Second Law limits on the performance of power cycles, heat pumps, and refrigerators 5) the temperature scale and its effect on calculating the second law limits for cycles 6) the Carnot cycle (power cycle or heat pump or refrigerator): what it is (the ...
Basic Thermodynamics - CERN Accelerator School
... number of possible microstates , the system will experimentally be found in the macrostate for which the number of microstates is the greater, thus having larger entropy. We consider, for example, a gas in a box, and assume that the gas is on one side of the box at some initial time corresponding ...
... number of possible microstates , the system will experimentally be found in the macrostate for which the number of microstates is the greater, thus having larger entropy. We consider, for example, a gas in a box, and assume that the gas is on one side of the box at some initial time corresponding ...
Ch. 17 Reaction Energy (Thermochemistry )
... 1. Is entropy increasing or decreasing with the following? a. Ice is melting _____________ b. Copper (II) Chloride is dissolved into water. _________ c. Gas is forming in a chemical reaction. _________ d. A reaction occurs between Magnesium and hydrochloric acid. _____________ e. Crystals of potassi ...
... 1. Is entropy increasing or decreasing with the following? a. Ice is melting _____________ b. Copper (II) Chloride is dissolved into water. _________ c. Gas is forming in a chemical reaction. _________ d. A reaction occurs between Magnesium and hydrochloric acid. _____________ e. Crystals of potassi ...
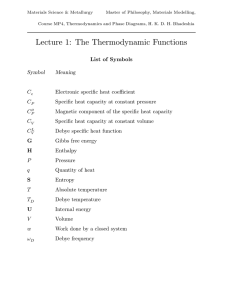
Thermodynamic functions - Phase Transformations Group
... which become increasingly violent as the temperature rises. These vibrations are elastic waves whose wavelengths can take discrete values consistent with the size of the sample. It follows that their energies are quantised, each quantum being called a phonon. The atoms need not all vibrate with the ...
... which become increasingly violent as the temperature rises. These vibrations are elastic waves whose wavelengths can take discrete values consistent with the size of the sample. It follows that their energies are quantised, each quantum being called a phonon. The atoms need not all vibrate with the ...
The Boltzmann distribution law and statistical thermodynamics
... Boltzmann distribution is related to the system’s free energy. That is the key to statistical thermodynamics. Together with a related but simpler observation about the connection between thermodynamic and mechanical energy, it amounts to having found the microscopic interpretation of the first and se ...
... Boltzmann distribution is related to the system’s free energy. That is the key to statistical thermodynamics. Together with a related but simpler observation about the connection between thermodynamic and mechanical energy, it amounts to having found the microscopic interpretation of the first and se ...
heat engine
... temperature, and all the rejected heat goes into a cold reservoir at a single temperature. Since the efficiency can only depend on the reservoir temperatures, the ratio of heats can only depend on those temperatures. QC QH ...
... temperature, and all the rejected heat goes into a cold reservoir at a single temperature. Since the efficiency can only depend on the reservoir temperatures, the ratio of heats can only depend on those temperatures. QC QH ...
Entropy in thermodynamics and information theory
There are close parallels between the mathematical expressions for the thermodynamic entropy, usually denoted by S, of a physical system in the statistical thermodynamics established by Ludwig Boltzmann and J. Willard Gibbs in the 1870s, and the information-theoretic entropy, usually expressed as H, of Claude Shannon and Ralph Hartley developed in the 1940s. Shannon, although not initially aware of this similarity, commented on it upon publicizing information theory in A Mathematical Theory of Communication.This article explores what links there are between the two concepts, and how far they can be regarded as connected.