NOTES on THERMODYNAMICS - University of Utah Physics
... matical structure is then constructed on the basis of these observations, which leads to a variety of useful concepts, and to testable relationships among various quantities. The laws of thermodynamics can only be justified by a more fundamental (microscopic) theory of nature. For example, statistica ...
... matical structure is then constructed on the basis of these observations, which leads to a variety of useful concepts, and to testable relationships among various quantities. The laws of thermodynamics can only be justified by a more fundamental (microscopic) theory of nature. For example, statistica ...
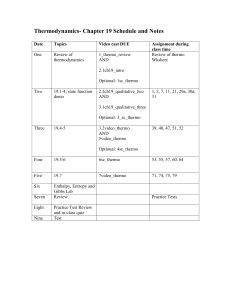
Schedule and sample problems
... (a) Predict the sign of the entropy change, ∆S, as the reaction proceeds to the right. Explain your prediction. (b) If the reaction spontaneously proceeds to the right, predict the sign of the enthalpy change, ∆H. Explain your prediction. (c) The direction in which the reaction spontaneously proceed ...
... (a) Predict the sign of the entropy change, ∆S, as the reaction proceeds to the right. Explain your prediction. (b) If the reaction spontaneously proceeds to the right, predict the sign of the enthalpy change, ∆H. Explain your prediction. (c) The direction in which the reaction spontaneously proceed ...
4. Classical Thermodynamics
... We need to start with a handful of definitions: • A system that is completely isolated from all outside influences is said to be contained in adiabatic walls. We will also refer to such systems as insulated. • Walls that are not adiabatic are said to be diathermal and two systems separated by a diat ...
... We need to start with a handful of definitions: • A system that is completely isolated from all outside influences is said to be contained in adiabatic walls. We will also refer to such systems as insulated. • Walls that are not adiabatic are said to be diathermal and two systems separated by a diat ...
Chapter 20
... (Va / Vd ) 1 . Combining the two expressions, one clearly obtains Vb / Vc Va / Vd , or Vb / Va Vc / Vd . This result implies | Q C | / | Q H | Tc / TH and Eq. (*). The Carnot efficiency is important because it is the highest possible efficiency any engine can reach if the highest possible te ...
... (Va / Vd ) 1 . Combining the two expressions, one clearly obtains Vb / Vc Va / Vd , or Vb / Va Vc / Vd . This result implies | Q C | / | Q H | Tc / TH and Eq. (*). The Carnot efficiency is important because it is the highest possible efficiency any engine can reach if the highest possible te ...
Thermodynamics
... change the total entropy of the universe. Irreversible processes increase the entropy of the universe. ...
... change the total entropy of the universe. Irreversible processes increase the entropy of the universe. ...
Thermodynamics of Skeletal Muscle During Cardiocirculatory Assist
... Bertalanffy [1] defined all living organisms as open systems, constantly exchanging energy and matter with the environment. For all we know, only living matter constitutes a system capable of opposing, albeit transitorily, this entropic tendency of the cosmos. Living systems are the only ones capabl ...
... Bertalanffy [1] defined all living organisms as open systems, constantly exchanging energy and matter with the environment. For all we know, only living matter constitutes a system capable of opposing, albeit transitorily, this entropic tendency of the cosmos. Living systems are the only ones capabl ...
The First and Second Laws of Thermodynamics
... degradation of energy during a process. As discussed later in this chapter, more of high-temperature energy can be converted to work, and thus it has a higher quality than the same amount of energy at a lower temperature. The second law of thermodynamics is also used in determining the theoretical l ...
... degradation of energy during a process. As discussed later in this chapter, more of high-temperature energy can be converted to work, and thus it has a higher quality than the same amount of energy at a lower temperature. The second law of thermodynamics is also used in determining the theoretical l ...
Chemistry 205 - Introductory General Chemistry
... the take home exams 1 week before they are due. There will be no lecture on the days the take home exams are due so that you may ask questions, however you should have at least attempted all of the problems by this time. Take home exams are due by 5 PM on the days listed. All work on take home exams ...
... the take home exams 1 week before they are due. There will be no lecture on the days the take home exams are due so that you may ask questions, however you should have at least attempted all of the problems by this time. Take home exams are due by 5 PM on the days listed. All work on take home exams ...
Word document format
... detail, as well as look at types of calculations involving these variables. In order to discuss these terms adequately, we need to define what is meant by a state function. A state function is a quantity whose value does not depend on the path used to measure the value. These quantities have upper c ...
... detail, as well as look at types of calculations involving these variables. In order to discuss these terms adequately, we need to define what is meant by a state function. A state function is a quantity whose value does not depend on the path used to measure the value. These quantities have upper c ...
Engines and the Second Law of Thermodynamics
... as an air conditioner in the summer, what would you expect its coefficient of performance to be, assuming all else stays the same? Qc = Qh – W = 4500 J – 1500 J = 3000 J And COP (refrigerator) = Qc/W = 3000/1500 = 2 Copyright © 2009 Pearson Education, Inc. ...
... as an air conditioner in the summer, what would you expect its coefficient of performance to be, assuming all else stays the same? Qc = Qh – W = 4500 J – 1500 J = 3000 J And COP (refrigerator) = Qc/W = 3000/1500 = 2 Copyright © 2009 Pearson Education, Inc. ...
General Theory of Finite Deformation
... x, I, Q, q The inequality consists of contributions to the entropy product due to three distinct processes: the deformation of the body, the heat conduction in the body, and the heat transfer between the body and reservoirs ...
... x, I, Q, q The inequality consists of contributions to the entropy product due to three distinct processes: the deformation of the body, the heat conduction in the body, and the heat transfer between the body and reservoirs ...
Equilibrium at constant temperature and pressure: Gibbs Free
... prevented. (In our block A/block B heat transfer experiment, the blocks did no work and were not allowed to exchange heat with their surroundings- only with one another). In such constant internal energy systems, it is straightforward to directly apply the fundamental equation for the entropy and th ...
... prevented. (In our block A/block B heat transfer experiment, the blocks did no work and were not allowed to exchange heat with their surroundings- only with one another). In such constant internal energy systems, it is straightforward to directly apply the fundamental equation for the entropy and th ...
Document
... So, in fact the two inflection points seen correspond to the deprotonation of the carboxylic group (at low pH) and then to the deprotonation of the amine group (at high pH). So, How can we estimate the fraction of these different species in solution? ...
... So, in fact the two inflection points seen correspond to the deprotonation of the carboxylic group (at low pH) and then to the deprotonation of the amine group (at high pH). So, How can we estimate the fraction of these different species in solution? ...
The first and second law of Thermodynamics - Ole Witt
... In the figure above is shown a machine M, which does the work W. The machine consumes the heat Q1 at temperature T1, and let out the heat Q2 at temperature T2 , performing the work W. Assuming that W > Wrev , we intend to show that it leads to a contradiction to the second, law in the Kelvin-Planck ...
... In the figure above is shown a machine M, which does the work W. The machine consumes the heat Q1 at temperature T1, and let out the heat Q2 at temperature T2 , performing the work W. Assuming that W > Wrev , we intend to show that it leads to a contradiction to the second, law in the Kelvin-Planck ...
lecture6
... vapour pressurePvap as the piston moves up, as long as both phases remain present. All that happens is that more water turns to steam, and the heat reservoir must supply the latent heat of vaporization, λ = 40.65 kilojoules per mole, in order to keep the temperature constant. The results of the pre ...
... vapour pressurePvap as the piston moves up, as long as both phases remain present. All that happens is that more water turns to steam, and the heat reservoir must supply the latent heat of vaporization, λ = 40.65 kilojoules per mole, in order to keep the temperature constant. The results of the pre ...
Document
... So, in fact the two inflection points seen correspond to the deprotonation of the carboxylic group (at low pH) and then to the deprotonation of the amine group (at high pH). So, How can we estimate the fraction of these different species in solution? ...
... So, in fact the two inflection points seen correspond to the deprotonation of the carboxylic group (at low pH) and then to the deprotonation of the amine group (at high pH). So, How can we estimate the fraction of these different species in solution? ...
Entropy in thermodynamics and information theory
There are close parallels between the mathematical expressions for the thermodynamic entropy, usually denoted by S, of a physical system in the statistical thermodynamics established by Ludwig Boltzmann and J. Willard Gibbs in the 1870s, and the information-theoretic entropy, usually expressed as H, of Claude Shannon and Ralph Hartley developed in the 1940s. Shannon, although not initially aware of this similarity, commented on it upon publicizing information theory in A Mathematical Theory of Communication.This article explores what links there are between the two concepts, and how far they can be regarded as connected.