T - Himastron
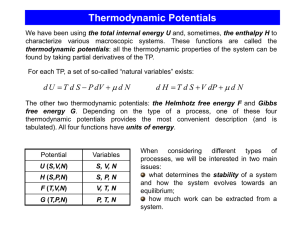
... approach. The entropy, along with V and N, determines the system’s energy U =U (S,V,N). Among the three variable, the entropy is the most difficult to control (the entropy-meters do not exist!). For an isolated system, we have to work with the entropy – it cannot be replaced with some other function ...
... approach. The entropy, along with V and N, determines the system’s energy U =U (S,V,N). Among the three variable, the entropy is the most difficult to control (the entropy-meters do not exist!). For an isolated system, we have to work with the entropy – it cannot be replaced with some other function ...
chapter 1
... measurement of biopotentials and electrode potentials, diagnostic methods using X-rays and ultrasound, optical and electron microscopy, radioisotope diagnostics, laser methods for diagnosis and treatment, methods of radiotherapy, differential scanning microcalorimetry, methods for separation of mole ...
... measurement of biopotentials and electrode potentials, diagnostic methods using X-rays and ultrasound, optical and electron microscopy, radioisotope diagnostics, laser methods for diagnosis and treatment, methods of radiotherapy, differential scanning microcalorimetry, methods for separation of mole ...
On violations of Le Chatelier`s principle for a temperature change in
... products tends to “resist” (by absorbing heat) the increase in temperature that the system is subject to. If the reaction is exothermic the reverse occurs. This qualitative principle is known by all chemists, and is extensively applied in the study of chemical reactions. It can be justified using a ...
... products tends to “resist” (by absorbing heat) the increase in temperature that the system is subject to. If the reaction is exothermic the reverse occurs. This qualitative principle is known by all chemists, and is extensively applied in the study of chemical reactions. It can be justified using a ...
Entropy and the Second and Third Laws of Thermodynamics
... rather that it will occur with high probability if any barrier to the change is overcome. For example, the transformation of a piece of wood to CO2 and H2O in the presence of oxygen is spontaneous, but it only occurs at elevated temperatures because an activation energy barrier must be overcome for ...
... rather that it will occur with high probability if any barrier to the change is overcome. For example, the transformation of a piece of wood to CO2 and H2O in the presence of oxygen is spontaneous, but it only occurs at elevated temperatures because an activation energy barrier must be overcome for ...
Nature of the anomalies in the supercooled liquid state of the mW
... model the supercooled liquid can no longer be equilibrated before it crystallizes and there is no sign of a liquid–liquid transition at supercooling. This raises the question: what is the origin of the anomalies in the mW model? In this work, we explain and reproduce these anomalies with a thermodyn ...
... model the supercooled liquid can no longer be equilibrated before it crystallizes and there is no sign of a liquid–liquid transition at supercooling. This raises the question: what is the origin of the anomalies in the mW model? In this work, we explain and reproduce these anomalies with a thermodyn ...
Chapter 17. Statistical thermodynamics 2: applications
... where B is the second virial coefficient and C is the third virial coefficient. • The total kinetic energy of a gas is the sum of the kinetic energies of the individual molecules. Therefore, even in a real gas the canonical partition function factorizes into a part arising from the kinetic energy, w ...
... where B is the second virial coefficient and C is the third virial coefficient. • The total kinetic energy of a gas is the sum of the kinetic energies of the individual molecules. Therefore, even in a real gas the canonical partition function factorizes into a part arising from the kinetic energy, w ...
Heat Engines, Entropy, and the Second Law of Thermodynamics P
... All natural processes are known to be irreversible. From the endless number of examples that could be selected, let us examine the adiabatic free expansion of a gas, which was already discussed in Section 20.6, and show that it cannot be reversible. The system that we consider is a gas in a thermall ...
... All natural processes are known to be irreversible. From the endless number of examples that could be selected, let us examine the adiabatic free expansion of a gas, which was already discussed in Section 20.6, and show that it cannot be reversible. The system that we consider is a gas in a thermall ...
Thermodynamics Of Chemical Processes
... Thermodynamics is one of the sciences on which Chemical Engineering is based upon. There are many definitions of the science of thermodynamics. The word originates from the Greek language. The Greek words thermo and dynamics mean heat and motion respectively. Thus, thermodynamics can be explained as ...
... Thermodynamics is one of the sciences on which Chemical Engineering is based upon. There are many definitions of the science of thermodynamics. The word originates from the Greek language. The Greek words thermo and dynamics mean heat and motion respectively. Thus, thermodynamics can be explained as ...
The first law of thermodynamics
... volume change of a gas at constant pressure. State the first law of thermodynamics. Identify the first law of thermodynamics as a statement of the principle of energy conservation. Describe the isochoric (isovolumetric), isobaric, isothermal and adiabatic changes of state of an ideal gas. ...
... volume change of a gas at constant pressure. State the first law of thermodynamics. Identify the first law of thermodynamics as a statement of the principle of energy conservation. Describe the isochoric (isovolumetric), isobaric, isothermal and adiabatic changes of state of an ideal gas. ...
Thermodynamics and Kinetics
... •The difference between the sum of the entropies of the products and the sum of the entropies of the reactants: In the above reaction, n and m are the coefficients of the products and the reactants in the balanced equation. As with H, entropies have been measured and tabulated. When: S > 0 disorde ...
... •The difference between the sum of the entropies of the products and the sum of the entropies of the reactants: In the above reaction, n and m are the coefficients of the products and the reactants in the balanced equation. As with H, entropies have been measured and tabulated. When: S > 0 disorde ...
20 Entropy and the Second Law of Thermodynamics
... confined by a closed stopcock to the left half of a thermally insulated container. If we open the stopcock, the gas rushes to fill the entire container, eventually reaching the final equilibrium state f shown in Fig. 20-1b. Unless the number of gas molecules is small (which is very hard to accomplis ...
... confined by a closed stopcock to the left half of a thermally insulated container. If we open the stopcock, the gas rushes to fill the entire container, eventually reaching the final equilibrium state f shown in Fig. 20-1b. Unless the number of gas molecules is small (which is very hard to accomplis ...
Chapter 3. Thermodynamics and Electrochemical Kinetics
... the maximum work, with no losses caused by irreversibilities, such as heat transfer through a finite temperature difference from T to To. But if a reversible heat engine were used to bridge the temperature difference, using T as the heat source and To as the heat sink, the heat transfer would become ...
... the maximum work, with no losses caused by irreversibilities, such as heat transfer through a finite temperature difference from T to To. But if a reversible heat engine were used to bridge the temperature difference, using T as the heat source and To as the heat sink, the heat transfer would become ...
Physics 1 Module 2: Thermodynamics
... • It is important to make a major distinction between internal energy and heat. Internal energy is all the energy of a system that is associated with its microscopic components—atoms and molecules— when viewed from a reference frame at rest with respect to the system. The last part of this sentence ...
... • It is important to make a major distinction between internal energy and heat. Internal energy is all the energy of a system that is associated with its microscopic components—atoms and molecules— when viewed from a reference frame at rest with respect to the system. The last part of this sentence ...
On the Foundations of Classical Thermodynamics, and the Tolman
... as auxiliary hypotheses which constrain the set of allowed dynamical processes of the more fundamental theory. While this macroscopic description might lack fundamental significance it does hold an epistemological high ground by virtue of being directly suggested to us by our senses. While it takes ...
... as auxiliary hypotheses which constrain the set of allowed dynamical processes of the more fundamental theory. While this macroscopic description might lack fundamental significance it does hold an epistemological high ground by virtue of being directly suggested to us by our senses. While it takes ...
Lecture Notes for Statistical Mechanics of Soft Matter
... such as phase space, temperature and entropy and many of the other statistical mechanical objects that will appear time and again in the remainder of this course. ...
... such as phase space, temperature and entropy and many of the other statistical mechanical objects that will appear time and again in the remainder of this course. ...
ee11042602mpt3.mov 110426ph423main3.mov Example of the
... This video clip starts at [00:30:44.09] and ends at [00:56:50:20] in the video ee1104262mp3.mov (InqScribed by Amanda Abbott) This narrative presents an example of an instructor engaging students in thinking conceptually about the partial derivatives they encounter in thermodynamic contexts. The ins ...
... This video clip starts at [00:30:44.09] and ends at [00:56:50:20] in the video ee1104262mp3.mov (InqScribed by Amanda Abbott) This narrative presents an example of an instructor engaging students in thinking conceptually about the partial derivatives they encounter in thermodynamic contexts. The ins ...
Chapter 1
... in chemical composition. o A pure substance can exist in more than one phase, but its chemical composition must be the same in each phase. ...
... in chemical composition. o A pure substance can exist in more than one phase, but its chemical composition must be the same in each phase. ...
Entropy in thermodynamics and information theory
There are close parallels between the mathematical expressions for the thermodynamic entropy, usually denoted by S, of a physical system in the statistical thermodynamics established by Ludwig Boltzmann and J. Willard Gibbs in the 1870s, and the information-theoretic entropy, usually expressed as H, of Claude Shannon and Ralph Hartley developed in the 1940s. Shannon, although not initially aware of this similarity, commented on it upon publicizing information theory in A Mathematical Theory of Communication.This article explores what links there are between the two concepts, and how far they can be regarded as connected.