calcijlation of elastic properties from thermodynamic equation of
... The variety of approaches involved in these studies demonstrates the need for a review of the methodology used to compute elastic wave speeds, in the spirit of an earlier review by Anderson et a1 (1968). Three broad areas bear on this topic: thermodynamic analysis, continuum mechanics, and solid sta ...
... The variety of approaches involved in these studies demonstrates the need for a review of the methodology used to compute elastic wave speeds, in the spirit of an earlier review by Anderson et a1 (1968). Three broad areas bear on this topic: thermodynamic analysis, continuum mechanics, and solid sta ...
Gibbs Free Energy and the Chemical Potential
... this function tells us the maximum amount of useful work that can be obtained from any process that occurs spontaneously at constant temperature and pressure, which is the condition under which most of biology occurs. The second reason is that the sign and magnitude of the change in Gibbs Free Energ ...
... this function tells us the maximum amount of useful work that can be obtained from any process that occurs spontaneously at constant temperature and pressure, which is the condition under which most of biology occurs. The second reason is that the sign and magnitude of the change in Gibbs Free Energ ...
Chapter 2. Thermodynamics
... Relations similar to Eqs (2.5) and (2.6) can be written for all types of phase transitions. Of particular importance are the transformations of crystalline solids from one type of crystal structure to another type. The concepts of heat and work are fundamentally different from the properties of a ma ...
... Relations similar to Eqs (2.5) and (2.6) can be written for all types of phase transitions. Of particular importance are the transformations of crystalline solids from one type of crystal structure to another type. The concepts of heat and work are fundamentally different from the properties of a ma ...
Optimizing natural gas fueling station reservoirs pressure based on
... alternative to other automobile fuels such as gasoline (petrol) and diesel1. Although there are huge available resources of natural gas, it has not been widely accepted as an alternative fuel to gasoline by most countries. The main obvious reason is low driving range of natural gas vehicles (NGV) wh ...
... alternative to other automobile fuels such as gasoline (petrol) and diesel1. Although there are huge available resources of natural gas, it has not been widely accepted as an alternative fuel to gasoline by most countries. The main obvious reason is low driving range of natural gas vehicles (NGV) wh ...
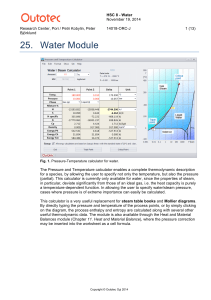
Water - HSC Chemistry 9
... difference is ~290 kJ and the process therefore endothermic. The entropy difference is ~1.079 kJ/°C and the process therefore generates entropy. The melting point of ice at 500 bar is calculated automatically, since the point is on the saturated liquid/solid curve, and it is roughly –4.5 °C. This pr ...
... difference is ~290 kJ and the process therefore endothermic. The entropy difference is ~1.079 kJ/°C and the process therefore generates entropy. The melting point of ice at 500 bar is calculated automatically, since the point is on the saturated liquid/solid curve, and it is roughly –4.5 °C. This pr ...
Continuous System Modeling - ETH
... • This bond graph is exceedingly beautiful ... It only has one drawback ... It is most certainly incorrect! ...
... • This bond graph is exceedingly beautiful ... It only has one drawback ... It is most certainly incorrect! ...
Thermodynamics with Chemical Engineering Applications
... First introduction of the Helmholtz and Gibbs free energy functions. First and Second Laws combined in four versions 8.3 Dependence of S, U, H, A, and G on T, p, and V. Maxwell’s relations 8.3.1 Entropy vs. p–V–T 8.3.2 Internal energy vs. p–V –T 8.3.3 Enthalpy vs. p–V –T 8.3.4 Helmholtz free energy ...
... First introduction of the Helmholtz and Gibbs free energy functions. First and Second Laws combined in four versions 8.3 Dependence of S, U, H, A, and G on T, p, and V. Maxwell’s relations 8.3.1 Entropy vs. p–V–T 8.3.2 Internal energy vs. p–V –T 8.3.3 Enthalpy vs. p–V –T 8.3.4 Helmholtz free energy ...
IV. Adiabatic Processes
... If a material undergoes a change in its physical state (e.g., its pressure, volume, or temperature) without any heat being added to it or withdrawn from it, the change is said to be adiabatic. Suppose that the initial state of a material is represented by the point A on the thermodynamic diagram bel ...
... If a material undergoes a change in its physical state (e.g., its pressure, volume, or temperature) without any heat being added to it or withdrawn from it, the change is said to be adiabatic. Suppose that the initial state of a material is represented by the point A on the thermodynamic diagram bel ...
A survey of statistical mechanics as it pertains to molecular simulation
... of statistical mechanics is devised to permit easy application of the postulates to nonisolated systems. This parallels the development of the formalism of thermodynamics, which begins by defining the entropy as a quantity that is maximized for an isolated system at equilibrium. Thermodynamics then ...
... of statistical mechanics is devised to permit easy application of the postulates to nonisolated systems. This parallels the development of the formalism of thermodynamics, which begins by defining the entropy as a quantity that is maximized for an isolated system at equilibrium. Thermodynamics then ...
thermodynamics type 1
... uniform throughout, made up of one phase only, pure liquid. solid, gas. A system is said to be heterogeneous if it consists of two or more phases, liquid in contact with vapour. STATE OF A SYSTEM : The state of a system is defined by a particular set of its measurable properties. For example, we can ...
... uniform throughout, made up of one phase only, pure liquid. solid, gas. A system is said to be heterogeneous if it consists of two or more phases, liquid in contact with vapour. STATE OF A SYSTEM : The state of a system is defined by a particular set of its measurable properties. For example, we can ...
Outline Introduction Introduction Gibbs Free Energy
... From practical point of view, P and T pair is the best choice because they are easy to control and measure. - For systems with constant pressure the most suitable state function is the Gibbs free energy G = H - TS On the other hand, V and T pair is easy to examine in statistical mechanics. - For ...
... From practical point of view, P and T pair is the best choice because they are easy to control and measure. - For systems with constant pressure the most suitable state function is the Gibbs free energy G = H - TS On the other hand, V and T pair is easy to examine in statistical mechanics. - For ...
Thermodynamics: Notes
... Therefore, since our temperature scale defines “hot” and “cold”, and a temperature difference determines heat flow, we can say that any return to or attainment of thermal equilibrium, as outlined above, can be used either to produce useful work, as in (3), or can be wastefully dissipated through a s ...
... Therefore, since our temperature scale defines “hot” and “cold”, and a temperature difference determines heat flow, we can say that any return to or attainment of thermal equilibrium, as outlined above, can be used either to produce useful work, as in (3), or can be wastefully dissipated through a s ...
Energy, Entropy and Exergy Concepts and Their Roles in Thermal
... These are only a meager number of engineering applications, and the study of thermodynamics is relevant to the analysis of a much wider range of processes and applications not only in engineering, but also in other fields of science. Therefore, a careful study of this topic is required to improve th ...
... These are only a meager number of engineering applications, and the study of thermodynamics is relevant to the analysis of a much wider range of processes and applications not only in engineering, but also in other fields of science. Therefore, a careful study of this topic is required to improve th ...
Chapter 2
... or a liquid) consisting of a single species is uniquely determined by its (number) density ρ = N/V , and temperature T , where N is the number of particles and V is the volume of the system. That is, the quantities P , T , and ρ are not independent, but are connected by a relation of the general ...
... or a liquid) consisting of a single species is uniquely determined by its (number) density ρ = N/V , and temperature T , where N is the number of particles and V is the volume of the system. That is, the quantities P , T , and ρ are not independent, but are connected by a relation of the general ...
THERMODYNAMICS LECTURE NOTES
... disregard the atomic nature of a substance and view it as a continuous, homogeneous matter with no holes, that is, a continuum. In Macroscopic approach of thermodynamics the substance is considered to be continuous whereas every matter actually comprises of myriads of molecules with intermolecular s ...
... disregard the atomic nature of a substance and view it as a continuous, homogeneous matter with no holes, that is, a continuum. In Macroscopic approach of thermodynamics the substance is considered to be continuous whereas every matter actually comprises of myriads of molecules with intermolecular s ...
1 CHAPTER 17 CHEMICAL THERMODYNAMICS 17.1 Equilibrium
... number of components and the number of degrees of freedom. But Whoa, there! We have been using several technical terms here: Phase, Component, Degrees of Freedom. We need to describe what these mean. The state of a system consisting of a single component in a single phase (for example a single gas – ...
... number of components and the number of degrees of freedom. But Whoa, there! We have been using several technical terms here: Phase, Component, Degrees of Freedom. We need to describe what these mean. The state of a system consisting of a single component in a single phase (for example a single gas – ...
- Philsci
... SM to TD. The surface effects generally do not smoothly vanish as the size of a system goes to infinity. Moreover the justification for ignoring surface effects in a given finite system is sensitive to the specifics of the system. (Griffith, 1972) But I shall not pursue this point for there is a cas ...
... SM to TD. The surface effects generally do not smoothly vanish as the size of a system goes to infinity. Moreover the justification for ignoring surface effects in a given finite system is sensitive to the specifics of the system. (Griffith, 1972) But I shall not pursue this point for there is a cas ...
Work Done On or By a Gas
... • Step 1: Gas in the system absorbs heat (Qin) from a heat source at a high temperature Th, expands and does work on surroundings without increasing internal energy • Step 2: The gas expands quickly and does work on surroundings, which causes the system to cool to a lower temperature, Tc. • Step 3: ...
... • Step 1: Gas in the system absorbs heat (Qin) from a heat source at a high temperature Th, expands and does work on surroundings without increasing internal energy • Step 2: The gas expands quickly and does work on surroundings, which causes the system to cool to a lower temperature, Tc. • Step 3: ...
Entropy in thermodynamics and information theory
There are close parallels between the mathematical expressions for the thermodynamic entropy, usually denoted by S, of a physical system in the statistical thermodynamics established by Ludwig Boltzmann and J. Willard Gibbs in the 1870s, and the information-theoretic entropy, usually expressed as H, of Claude Shannon and Ralph Hartley developed in the 1940s. Shannon, although not initially aware of this similarity, commented on it upon publicizing information theory in A Mathematical Theory of Communication.This article explores what links there are between the two concepts, and how far they can be regarded as connected.