Boundless Study Slides
... Boundless content pre-organized to match the assigned textbook. This platform gives educators the tools they need to assign readings and assessments, monitor student activity, and lead their classes with pre-made ...
... Boundless content pre-organized to match the assigned textbook. This platform gives educators the tools they need to assign readings and assessments, monitor student activity, and lead their classes with pre-made ...
Thermodynamics: Four Laws That Move the Universe
... is the crucial link between temperature and thermal energy. It is a way to quantify how many different ways there are to distribute energy, and as you will learn, it is the foundation for the second law of thermodynamics. By understanding entropy, you will gain an intuitive sense for the connectedne ...
... is the crucial link between temperature and thermal energy. It is a way to quantify how many different ways there are to distribute energy, and as you will learn, it is the foundation for the second law of thermodynamics. By understanding entropy, you will gain an intuitive sense for the connectedne ...
Basic Thermodynamics - Alpha College of Engineering
... sloped 300 from the horizontal. If the velocity of the car is to remain constant during climbing, determine the additional power that must be delivered by the engine. [04 Marks, Jun-2015] 4. A centrifugal pump delivers 50 kg of water per second. The inlet and outlet pressure are 1 bar and 4.2 bar re ...
... sloped 300 from the horizontal. If the velocity of the car is to remain constant during climbing, determine the additional power that must be delivered by the engine. [04 Marks, Jun-2015] 4. A centrifugal pump delivers 50 kg of water per second. The inlet and outlet pressure are 1 bar and 4.2 bar re ...
Chemical Thermodynamics
... negative, corresponding to work done by a system on its surroundings. Conversely, when a gas is compressed by an external pressure, ΔV < 0 and the work is positive because work is being done on a system by its surroundings. Suppose, for example, that the system under study is a mass of steam heated ...
... negative, corresponding to work done by a system on its surroundings. Conversely, when a gas is compressed by an external pressure, ΔV < 0 and the work is positive because work is being done on a system by its surroundings. Suppose, for example, that the system under study is a mass of steam heated ...
Thermodynamic Cycles Knowledge Check
... Introduction We have not yet discussed processes performed by gases as we have focused on the steam cycle, yet many applications of the use of gases are occurring all the time during plant operation. The compression of a gas results in different final states than the compression of a saturated vapo ...
... Introduction We have not yet discussed processes performed by gases as we have focused on the steam cycle, yet many applications of the use of gases are occurring all the time during plant operation. The compression of a gas results in different final states than the compression of a saturated vapo ...
Thermodynamic Cycles Knowledge Check
... Introduction We have not yet discussed processes performed by gases as we have focused on the steam cycle, yet many applications of the use of gases are occurring all the time during plant operation. The compression of a gas results in different final states than the compression of a saturated vapo ...
... Introduction We have not yet discussed processes performed by gases as we have focused on the steam cycle, yet many applications of the use of gases are occurring all the time during plant operation. The compression of a gas results in different final states than the compression of a saturated vapo ...
Thermodynamic Cycles Knowledge Check
... Introduction We have not yet discussed processes performed by gases as we have focused on the steam cycle, yet many applications of the use of gases are occurring all the time during plant operation. The compression of a gas results in different final states than the compression of a saturated vapo ...
... Introduction We have not yet discussed processes performed by gases as we have focused on the steam cycle, yet many applications of the use of gases are occurring all the time during plant operation. The compression of a gas results in different final states than the compression of a saturated vapo ...
Intermediate Physical Chemistry (CHEM2503)
... q the total number of states. Therefore, the molecular partition function gives an indication of the average number of states that are thermally accessible to a molecule at the temperature of the system. The larger the value of the partition function is, the more the number of thermally accessible ...
... q the total number of states. Therefore, the molecular partition function gives an indication of the average number of states that are thermally accessible to a molecule at the temperature of the system. The larger the value of the partition function is, the more the number of thermally accessible ...
Thermodynamics Theory + Questions.0001
... letters as the symbols). e.g., Volume, Mass (V, M). If mass is increased, the value of extensive property also increases. e.g., volume V, internal energy U, enthalpy H, entropy S, etc. Specific property: It is a special case of an intensive property. It is the value of an extensive property per unit ...
... letters as the symbols). e.g., Volume, Mass (V, M). If mass is increased, the value of extensive property also increases. e.g., volume V, internal energy U, enthalpy H, entropy S, etc. Specific property: It is a special case of an intensive property. It is the value of an extensive property per unit ...
Thermodynamics & Statistical Mechanics:
... interviewed. I know that even if the polling was done without bias, which is extremely unlikely, the laws of statistics say that there is a intrinsic error of order one over the square root of the number of people questioned. It follows that if a thousand people were interviewed, which is a typical ...
... interviewed. I know that even if the polling was done without bias, which is extremely unlikely, the laws of statistics say that there is a intrinsic error of order one over the square root of the number of people questioned. It follows that if a thousand people were interviewed, which is a typical ...
Enthalpy, Entropy, Mollier Diagram and Steam
... transformation of the Mollier diagram to the psychrometric chart on the basis of geometric manipulation. This relationship between Mollier diagram and the psychrometric chart is apparent from the fact that both involve critical thermodynamic properties such as enthalpy, temperature, sensible heat, l ...
... transformation of the Mollier diagram to the psychrometric chart on the basis of geometric manipulation. This relationship between Mollier diagram and the psychrometric chart is apparent from the fact that both involve critical thermodynamic properties such as enthalpy, temperature, sensible heat, l ...
Water Vapor and Mechanical Work: A Comparison of
... cycle often overestimates the conversion of internal energy into kinetic energy in the presence of water vapor. To better assess the role of water vapor, a novel thermodynamic cycle is introduced here: the steam cycle. A steam cycle transports water from a moist source to a dry sink; in doing so, it ...
... cycle often overestimates the conversion of internal energy into kinetic energy in the presence of water vapor. To better assess the role of water vapor, a novel thermodynamic cycle is introduced here: the steam cycle. A steam cycle transports water from a moist source to a dry sink; in doing so, it ...
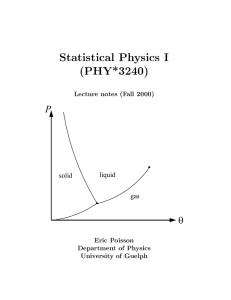
book - University of Guelph Physics
... The existence of the relation g(P, V ) = θ for isotherms, inferred empirically above, can also be justified by rigourous mathematics. We will now go through this argument. This will serve to illustrate a major theme of thermodynamics: Simple physical ideas (such as the zeroth law) can go very far wh ...
... The existence of the relation g(P, V ) = θ for isotherms, inferred empirically above, can also be justified by rigourous mathematics. We will now go through this argument. This will serve to illustrate a major theme of thermodynamics: Simple physical ideas (such as the zeroth law) can go very far wh ...
S15--AP Phys Q4--Heat-Thermo Ch13_14_15
... transferred to or from a system by work is equivalent to which of the following? a. volume change b. pressure change c. entropy change d. internal energy change 3. Which of the following is not a widely used temperature scale? a. Kelvin b. Celsius c. Joule d. Fahrenheit 4. An ideal gas system underg ...
... transferred to or from a system by work is equivalent to which of the following? a. volume change b. pressure change c. entropy change d. internal energy change 3. Which of the following is not a widely used temperature scale? a. Kelvin b. Celsius c. Joule d. Fahrenheit 4. An ideal gas system underg ...
Statistical Mechanics
... Figure 1.7: Isolated system divided into two parts Consider an isolated system (no heat or work in or out) that we arbitrarily divide into two parts (see Fig. 1.7). Call the total volume V = V1 + V2 . Similarly, the total internal energy is U = U1 + U2 . These thermodynamic variables are said to be ...
... Figure 1.7: Isolated system divided into two parts Consider an isolated system (no heat or work in or out) that we arbitrarily divide into two parts (see Fig. 1.7). Call the total volume V = V1 + V2 . Similarly, the total internal energy is U = U1 + U2 . These thermodynamic variables are said to be ...
Chapter 19 Thermodynamics - Farmingdale State College
... which is the work done on the gas by the external agent. If the compression takes place very slowly, the constant external pressure exerted by the piston on the gas is equal to the internal pressure exerted by the gas throughout the process. Thus, equation 19.3 can also be interpreted as the work do ...
... which is the work done on the gas by the external agent. If the compression takes place very slowly, the constant external pressure exerted by the piston on the gas is equal to the internal pressure exerted by the gas throughout the process. Thus, equation 19.3 can also be interpreted as the work do ...
Heat Engines, Entropy, and the Second Law of Thermodynamics
... It is impossible to construct a cyclical machine whose sole effect is to transfer energy continuously by heat from one object to another object at a higher temperature without the input of energy by work. In simpler terms, energy does not transfer spontaneously by heat from a cold object to a hot ob ...
... It is impossible to construct a cyclical machine whose sole effect is to transfer energy continuously by heat from one object to another object at a higher temperature without the input of energy by work. In simpler terms, energy does not transfer spontaneously by heat from a cold object to a hot ob ...
The last chapter of David Albert`s Time and Chance - Philsci
... those of the interventions; it is for this reason that the account requires that every phase point p has high (intervention-driven) probability to evolve in the right way. If we had required only that mc-most p so evolve, we would then need to explain the connection between this (mathematical) fact ...
... those of the interventions; it is for this reason that the account requires that every phase point p has high (intervention-driven) probability to evolve in the right way. If we had required only that mc-most p so evolve, we would then need to explain the connection between this (mathematical) fact ...
Constructor Theory of Thermodynamics
... i.e., their domain of applicability is limited to a certain scale, which is never properly defined. Since my approach improves particularly on the axiomatic formulation of thermodynamics, I shall refer to it (in its most recent formulation, by Lieb and Yngvason [4]) to illustrate the problem of scal ...
... i.e., their domain of applicability is limited to a certain scale, which is never properly defined. Since my approach improves particularly on the axiomatic formulation of thermodynamics, I shall refer to it (in its most recent formulation, by Lieb and Yngvason [4]) to illustrate the problem of scal ...
Entropy in thermodynamics and information theory
There are close parallels between the mathematical expressions for the thermodynamic entropy, usually denoted by S, of a physical system in the statistical thermodynamics established by Ludwig Boltzmann and J. Willard Gibbs in the 1870s, and the information-theoretic entropy, usually expressed as H, of Claude Shannon and Ralph Hartley developed in the 1940s. Shannon, although not initially aware of this similarity, commented on it upon publicizing information theory in A Mathematical Theory of Communication.This article explores what links there are between the two concepts, and how far they can be regarded as connected.