Statistical - Jordan University of Science and Technology
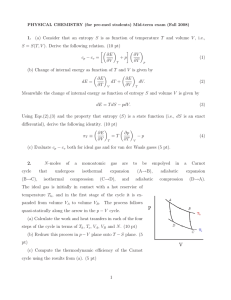
... position and let n be the number of atoms occupying interstitial sites in equilibrium. a) what is the internal energy of the system. b) What is the total energy. Give an asymptotic formula when n>> 1? c) In equilibrium at temperature T, how many such defects are there in the solid. i.e what is n? as ...
... position and let n be the number of atoms occupying interstitial sites in equilibrium. a) what is the internal energy of the system. b) What is the total energy. Give an asymptotic formula when n>> 1? c) In equilibrium at temperature T, how many such defects are there in the solid. i.e what is n? as ...
HERE - MRS. STOTTS CHEMISTRY
... What is the likelihood they both will end up there? (1/2)2 If one mole is used? (1/2)6.02×1023! (No chance!) Gases _______________ expand to fill the volume given. Most probable arrangement of molecules: approximately ____________molecules in each side Statistical Thermodynamics-10 Thermodynamics lo ...
... What is the likelihood they both will end up there? (1/2)2 If one mole is used? (1/2)6.02×1023! (No chance!) Gases _______________ expand to fill the volume given. Most probable arrangement of molecules: approximately ____________molecules in each side Statistical Thermodynamics-10 Thermodynamics lo ...
Problem Set 5 - 2004
... You should read Chapter 5 of Thermal Physics by C.B.P. Finn, if you have not already. (1) For each of the following processes, state whether the entropy is increasing, decreasing or staying the same. (i) A piston filled with an ideal gas is surrounded by very good thermal insulation. It is compresse ...
... You should read Chapter 5 of Thermal Physics by C.B.P. Finn, if you have not already. (1) For each of the following processes, state whether the entropy is increasing, decreasing or staying the same. (i) A piston filled with an ideal gas is surrounded by very good thermal insulation. It is compresse ...
The Second Law of Thermodynamics
... The entropy of an isolated system increases in any irreversible process and is unaltered in any reversible process. This is the principle of increasing entropy. The fact that the entropy of an isolated system can never decrease in a process provides a direction for the sequence of natural events. ...
... The entropy of an isolated system increases in any irreversible process and is unaltered in any reversible process. This is the principle of increasing entropy. The fact that the entropy of an isolated system can never decrease in a process provides a direction for the sequence of natural events. ...
Thermodynamic principles. - med.muni
... – Thermodynamic system: A region of space bounded by arbitrary surfaces which delineate the portion of the universe we are interested in – Isolated system: one which cannot exchange particles or energy with its environment. – Open system: one which can exchanges both particles and energy with its en ...
... – Thermodynamic system: A region of space bounded by arbitrary surfaces which delineate the portion of the universe we are interested in – Isolated system: one which cannot exchange particles or energy with its environment. – Open system: one which can exchanges both particles and energy with its en ...
Internal energy is a characteristic of a given state – it is the same no
... limited situations where it doesn’t increase. (This is equivalent to second law of thermodynamics.) ...
... limited situations where it doesn’t increase. (This is equivalent to second law of thermodynamics.) ...
Day 58 - Tahoma
... effuse into vessel B, but it will not spontaneously return to vessel A. Processes that are spontaneous in ...
... effuse into vessel B, but it will not spontaneously return to vessel A. Processes that are spontaneous in ...
9. Entropy 2nd and 3rd laws/ Thermodynamic processes / Droplet
... 9.1 Entropy in second and third laws of thermodynamics (2pts) 1. Explain the statistical definition of entropy (4pts) 2. Consider a “thermodynamic system” of two dices and let the energy of a certain throw (state of the system) be the sum of the two values of the dices. Calculate the respective entr ...
... 9.1 Entropy in second and third laws of thermodynamics (2pts) 1. Explain the statistical definition of entropy (4pts) 2. Consider a “thermodynamic system” of two dices and let the energy of a certain throw (state of the system) be the sum of the two values of the dices. Calculate the respective entr ...
As a system asymptotically approaches absolute zero of
... and the entropy of the system asymptotically approaches a minimum value; also stated as: "the entropy of all systems and of all states of a system is smallest at absolute zero" or equivalently "it is impossible to reach the absolute zero of temperature by any finite number of processes." Absolute ze ...
... and the entropy of the system asymptotically approaches a minimum value; also stated as: "the entropy of all systems and of all states of a system is smallest at absolute zero" or equivalently "it is impossible to reach the absolute zero of temperature by any finite number of processes." Absolute ze ...
entropy - Helios
... Entropy Change Imagine now a simple idealized system consisting of a box of gas in contact with a heat reservoir ...
... Entropy Change Imagine now a simple idealized system consisting of a box of gas in contact with a heat reservoir ...
Chapter Entropy Statistics
... δQ = dU + PdV Since V is constant, so δQ = dU Since δQ is infinitesimally small so the system is assumed to be in most probable state ds = δQ /T ...
... δQ = dU + PdV Since V is constant, so δQ = dU Since δQ is infinitesimally small so the system is assumed to be in most probable state ds = δQ /T ...
SCH 303: THERMODYNAMICS II AND PHASE EQUILIBRIA Course
... system reversibly as opposed to irreversibly To define systems properties such as U, H, S as path dependent or exact differentials and the consequences. To give thermodynamic determinants of spontaneous process To evaluate the entropy of systems under different conditions To define the Gibbs and Hel ...
... system reversibly as opposed to irreversibly To define systems properties such as U, H, S as path dependent or exact differentials and the consequences. To give thermodynamic determinants of spontaneous process To evaluate the entropy of systems under different conditions To define the Gibbs and Hel ...
8 second law of thermodynamics : states spontaneous process is
... up to produce a liquid with an entropy increased of 10.5J .At its boiling point the liquid structure is completely destroyed to form the completely random gaseous phase and the entropy increase by 96j/k. we know that the change in the state of ordere of the system at the boiling point is much greate ...
... up to produce a liquid with an entropy increased of 10.5J .At its boiling point the liquid structure is completely destroyed to form the completely random gaseous phase and the entropy increase by 96j/k. we know that the change in the state of ordere of the system at the boiling point is much greate ...
After separation into groups students got working sheets and
... One student presented his homework which included example of irreverzibile process. Other students thought of more natural irreversible processes. After showing students PowerPoint presentation about entropy they defined entropy as general law. ...
... One student presented his homework which included example of irreverzibile process. Other students thought of more natural irreversible processes. After showing students PowerPoint presentation about entropy they defined entropy as general law. ...
Entropy and the end of it all
... The waiting time is called a Poincare cycle. Finally, no one knows the answer to these dilemma's because we now realize that Newtonian mechanics is only partly right. You have to study quantum mechanics if you really want to know how things work or at least how we ...
... The waiting time is called a Poincare cycle. Finally, no one knows the answer to these dilemma's because we now realize that Newtonian mechanics is only partly right. You have to study quantum mechanics if you really want to know how things work or at least how we ...
Entropy in thermodynamics and information theory
There are close parallels between the mathematical expressions for the thermodynamic entropy, usually denoted by S, of a physical system in the statistical thermodynamics established by Ludwig Boltzmann and J. Willard Gibbs in the 1870s, and the information-theoretic entropy, usually expressed as H, of Claude Shannon and Ralph Hartley developed in the 1940s. Shannon, although not initially aware of this similarity, commented on it upon publicizing information theory in A Mathematical Theory of Communication.This article explores what links there are between the two concepts, and how far they can be regarded as connected.