Apr25_2_Duthil - CERN Accelerator School
... a thermodynamic system is in thermodynamic equilibrium when there are no net flows of matter or of energy, no phase changes, and no unbalanced potentials (or driving forces) within the system. A system that is in thermodynamic equilibrium experiences no changes when it is isolated from its surroundi ...
... a thermodynamic system is in thermodynamic equilibrium when there are no net flows of matter or of energy, no phase changes, and no unbalanced potentials (or driving forces) within the system. A system that is in thermodynamic equilibrium experiences no changes when it is isolated from its surroundi ...
First Law of Thermodynamics - Derry Area School District
... When you look at large systems like the ideal gas with 1023 particles, the most likely macrostate – described by p, V, and T and obeying the ideal gas law – has so many microstates associated with it that it’s the only one you have any chance of observing. • When you allow two systems at different t ...
... When you look at large systems like the ideal gas with 1023 particles, the most likely macrostate – described by p, V, and T and obeying the ideal gas law – has so many microstates associated with it that it’s the only one you have any chance of observing. • When you allow two systems at different t ...
Chemical Thermodynamics John Murrell Introduction
... like water (if this were not so then molecules such as NaCl would not dissociate to ions in water). For solvated ions one uses a completely different standard from that used for neutral molecules, and this is that the enthalpy of formation of H+(aq) is zero. Liquids and solids have lower energies t ...
... like water (if this were not so then molecules such as NaCl would not dissociate to ions in water). For solvated ions one uses a completely different standard from that used for neutral molecules, and this is that the enthalpy of formation of H+(aq) is zero. Liquids and solids have lower energies t ...
Biological Thermodynamics
... The First Law of thermodynamics The Energy is conserved The total energy of a system and its surroundings is constant In any physical or chemical change, the total amount of energy in the universe remains constant, although the form of the energy may change. ...
... The First Law of thermodynamics The Energy is conserved The total energy of a system and its surroundings is constant In any physical or chemical change, the total amount of energy in the universe remains constant, although the form of the energy may change. ...
slides - Biology Courses Server
... The “Classical” Definition of Entropy, S For two states for which temperature remains constant during the reversible process of converting one to the other: qrev T For two states separated by a reversible process for which the temperature does not stay constant: ∆S = ...
... The “Classical” Definition of Entropy, S For two states for which temperature remains constant during the reversible process of converting one to the other: qrev T For two states separated by a reversible process for which the temperature does not stay constant: ∆S = ...
Max Planck: the reluctant revolutionary Helge Kaigh, Physics World
... probabilistic) interpretation that Ludwig Boltzmann had originally proposed back in 1872 and expanded in 1877. According to Boltzmann’s molecularmechanical interpretation, the entropy of a system is the collective result of molecular motions. The second law is valid only in a statistical sense. Bolt ...
... probabilistic) interpretation that Ludwig Boltzmann had originally proposed back in 1872 and expanded in 1877. According to Boltzmann’s molecularmechanical interpretation, the entropy of a system is the collective result of molecular motions. The second law is valid only in a statistical sense. Bolt ...
Chapter Two The Thermodynamic Laws
... "Heat cannot of itself pass from a colder to a hotter body." This statement implies an inequality of the heat transfer between a hot body and a cold body. Heat transfer from a hot body to a cold body can spontaneously occur. However, heat transfer in the reversed direction can not happen without the ...
... "Heat cannot of itself pass from a colder to a hotter body." This statement implies an inequality of the heat transfer between a hot body and a cold body. Heat transfer from a hot body to a cold body can spontaneously occur. However, heat transfer in the reversed direction can not happen without the ...
Thermo applications
... if the enthalpies of all the chemical species are calculated relative to the same reference state, namely that of the elements in their standard states at 25°C and 1 atm. By choosing the elemental reference state, the difference in enthalpy can be calculated without having to take into account any o ...
... if the enthalpies of all the chemical species are calculated relative to the same reference state, namely that of the elements in their standard states at 25°C and 1 atm. By choosing the elemental reference state, the difference in enthalpy can be calculated without having to take into account any o ...
The Helmholtz Function
... One often introduces the notion of canonically conjugate pairs, one variable being extensive and the other intensive. For example (V, -P), (S, T). ...
... One often introduces the notion of canonically conjugate pairs, one variable being extensive and the other intensive. For example (V, -P), (S, T). ...
Statistical Thermodynamics and Stochastic The
... its development the mathematics of nonlinear processes, the so-called nonlinear dynamics. The great pioneers of nonlinear dynamics in the 19th century were Helmholtz, Rayleigh, Poincare and Lyapunov. John William Rayleigh (1842-1919) is the founder of the theory of nonlinear oscillations. Many appli ...
... its development the mathematics of nonlinear processes, the so-called nonlinear dynamics. The great pioneers of nonlinear dynamics in the 19th century were Helmholtz, Rayleigh, Poincare and Lyapunov. John William Rayleigh (1842-1919) is the founder of the theory of nonlinear oscillations. Many appli ...
2. Local equilibrium thermodynamics.
... static macroscopic equilibrium states of small local regions. The independent state variables of a small local region are only those of classical thermodynamics. Generalized or extended thermodynamics Like local equilibrium thermodynamics, generalized or extended thermodynamics also is concerned wi ...
... static macroscopic equilibrium states of small local regions. The independent state variables of a small local region are only those of classical thermodynamics. Generalized or extended thermodynamics Like local equilibrium thermodynamics, generalized or extended thermodynamics also is concerned wi ...
NOTES ON THERMODYNAMIC FORMALISM
... γ. Since any loop in the P V -plane can be approximated by a sum of Carnot cycles, δQ/T must be an exact differential, so that we can write it in the form δQ dS := T The quantity S (a priori defined only up to an additive constant) is called the entropy of the system, and is seen by this reasoning t ...
... γ. Since any loop in the P V -plane can be approximated by a sum of Carnot cycles, δQ/T must be an exact differential, so that we can write it in the form δQ dS := T The quantity S (a priori defined only up to an additive constant) is called the entropy of the system, and is seen by this reasoning t ...
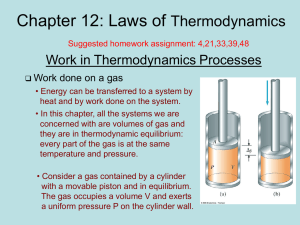
chapter12
... violate the First Law, giving out more energy than was put into the machine • Perpetual motion machines of the second type would violate the Second Law, possibly by no exhaust • Perpetual motion machines will never be invented ...
... violate the First Law, giving out more energy than was put into the machine • Perpetual motion machines of the second type would violate the Second Law, possibly by no exhaust • Perpetual motion machines will never be invented ...
Carnot Cycle. Heat Engines. Refrigerators.
... The first law says you can not get efficiency greater than unity. The second law forbids an efficiency of unity – not all energy absorbed as heat can be converted into work. Better efficiency comes by making the ratio TTc as small as possible. We can summarize the effect h of the second law as: “You ...
... The first law says you can not get efficiency greater than unity. The second law forbids an efficiency of unity – not all energy absorbed as heat can be converted into work. Better efficiency comes by making the ratio TTc as small as possible. We can summarize the effect h of the second law as: “You ...
Meaning of Entropy in Classical Thermodynamics
... One needs to understand irreversible processes in order to understand reversible ones. Truesdell is looking for a mathematical expression for irreversible processes since he criticizes Thermodynamics as a subject with an unusually high ratio of words to equations. In modern thermodynamics textbooks, ...
... One needs to understand irreversible processes in order to understand reversible ones. Truesdell is looking for a mathematical expression for irreversible processes since he criticizes Thermodynamics as a subject with an unusually high ratio of words to equations. In modern thermodynamics textbooks, ...
The laws of thermodynamics - Assets
... pressure, mass density, heat capacity, etc., are the properties of main interest. The number of atoms or molecules contained, and hence the volume of the system, must be sufficiently large so that the conditions on the surfaces of the system do not affect the macroscopic properties significantly. From ...
... pressure, mass density, heat capacity, etc., are the properties of main interest. The number of atoms or molecules contained, and hence the volume of the system, must be sufficiently large so that the conditions on the surfaces of the system do not affect the macroscopic properties significantly. From ...
Entropy in thermodynamics and information theory
There are close parallels between the mathematical expressions for the thermodynamic entropy, usually denoted by S, of a physical system in the statistical thermodynamics established by Ludwig Boltzmann and J. Willard Gibbs in the 1870s, and the information-theoretic entropy, usually expressed as H, of Claude Shannon and Ralph Hartley developed in the 1940s. Shannon, although not initially aware of this similarity, commented on it upon publicizing information theory in A Mathematical Theory of Communication.This article explores what links there are between the two concepts, and how far they can be regarded as connected.