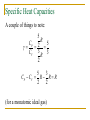* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Thermodynamics
Thermoregulation wikipedia , lookup
Entropy in thermodynamics and information theory wikipedia , lookup
Conservation of energy wikipedia , lookup
Non-equilibrium thermodynamics wikipedia , lookup
Thermal conductivity wikipedia , lookup
Dynamic insulation wikipedia , lookup
Temperature wikipedia , lookup
Internal energy wikipedia , lookup
Thermal radiation wikipedia , lookup
Calorimetry wikipedia , lookup
Heat exchanger wikipedia , lookup
Heat capacity wikipedia , lookup
R-value (insulation) wikipedia , lookup
First law of thermodynamics wikipedia , lookup
Copper in heat exchangers wikipedia , lookup
Countercurrent exchange wikipedia , lookup
Extremal principles in non-equilibrium thermodynamics wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Heat equation wikipedia , lookup
Heat transfer physics wikipedia , lookup
Heat transfer wikipedia , lookup
Thermal conduction wikipedia , lookup
Thermodynamic system wikipedia , lookup
Second law of thermodynamics wikipedia , lookup
Thermodynamics Chapter 15 Expectations After this chapter, students will: Recognize and apply the four laws of thermodynamics Understand what is meant by thermodynamic terms Recognize and distinguish among four kinds of thermal processes Distinguish between reversible and irreversible processes Expectations After this chapter, students will: Analyze the properties and operations of heat engines Calculate the changes in entropy associated with thermal processes Thermodynamics: Some Fundamentals Thermodynamics is the study of the economy of heat energy and mechanical work, in the context of the ideal gas. Understanding thermodynamic concepts and mathematical relationships depends on understanding some terms. Systems and Their Boundaries In mechanics, we introduced the idea of the system: a user-defined collection of objects. In thermodynamics, “system” still means that. However, we add the notion that the system will usually include some definite amount of a fluid – typically, an ideal gas. It might also include other elements, such as the fluid’s container. It’s always important to be clear about what’s in the system, and what isn’t. Systems and Their Boundaries Once we have decided what the system is, we can say that the surroundings consists of everything that isn’t the system. The system is separated from its surroundings by walls. If the walls do not allow heat to pass through them, they are adiabatic walls. If heat can pass freely through the walls, we call them diathermal walls. The State of a System The characteristics that tell us what we want to know about a system define its condition, or state. If the system consists of some amount of an ideal gas, we’ll be interested in the temperature, pressure, volume, and mass of the gas. Those quantities are the state variables of the system. Common Sense: 0th Law of Thermodynamics Suppose we have three systems: A, B, and C. Suppose that A is in thermal equilibrium with C. Suppose also that B is in thermal equilibrium with C. Then: A is in thermal equilibrium with B, and no heat will flow between them if they are in contact. (C is intended to be a thermometer.) Energy Conservation: 1st Law of Thermodynamics Mathematical statement: DU U f U 0 Q W What does this mean? DU is the change in the system’s internal energy Uf is the system’s final internal energy U0 is the system’s initial internal energy Q is the heat added to the system W is the work done by the system Energy Conservation: 1st Law of Thermodynamics DU U f U 0 Q W Think of U as being the system’s bank balance, and think of work and energy and heat as being forms of cash. What we’ve said is that the change in the system’s balance is its income (heat) minus its spending (work). Thermal Processes A process is how the system, and its surroundings, change from one state to another. We’re going to consider four special cases of thermal processes. These special cases help us to understand what the laws of thermodynamics tell us about systems and their properties. Thermal Processes In every case, we assume that the process occurs “slowly.” The technical term for “occurs slowly” is quasi-static. What does “slowly” mean? It means that the system has time to mix during the process. At all times, we consider the temperature and the pressure of the system to be uniform (the same in all places throughout the system). Isobaric Process Isobaric: the pressure of the system is constant. The result first: W P DV PV f V0 Why should this be? Isobaric Process Consider an ideal gas to which heat is added. W F S PAS But A·S = DV = Vf – Vi : W P DV PV f Vi Isobaric Process This process can be represented graphically, plotting pressure vs. volume. Notice that the work is the area under this plot (P times DV). Isochoric Process Isochoric: the volume of the system is constant. The result: W 0 DU Q W Q Why? If volume is constant, nothing moves. No motion, no work. Any heat added to the system only changes its internal energy. Isochoric Process Pressure-volume plot for an isochoric process: No area under the plot means no work is done. Isothermal Process Isothermal: the temperature of the system is constant. Use the ideal gas equation to write P as a function of V: PV nRT nRT P V Because P is not constant, W P DV Isothermal Process The work is still the area under the isotherm plot. To calculate it, we integrate: Vi nRT W dV V Vf (natural log) Vf W nRT ln Vi Adiabatic Process In an adiabatic process, no heat enters or leaves the system: Q = 0. The First Law becomes: DU Q W W But, as we learned in chapter 14, 3 U nRT 2 (for a monatomic ideal gas). Adiabatic Process 3 3 If U nRT , then DU nRDT 2 2 and DU Q W W 3 3 so W nRDT nRTi T f 2 2 3 W nR Ti T f 2 (monatomic ideal gas) Adiabatic Process Notice that the adiabatic curve is different from both isotherms (corresponding to the initial and final temperatures). Its equation is: PiVi Pf V f cP where cV specific heat capacity @ constant pressure specific heat capacity @ constant volume Specific Heat Capacities Define a molar specific heat capacity, C, for an ideal gas: Q = C n DT (SI units: J / mol·K) First Law: DU Q W Q DU W At constant pressure (isobaric): W P DV PV f Vi Specific Heat Capacities Substitute for Vi and Vf from the ideal gas equation: nRT V P W PV f Vi nRT f Ti 3 For a monatomic ideal gas: DU nRT f Ti 2 Substitute: 3 5 Q DU W nRT f Ti nRT f Ti nR T f Ti 2 2 Specific Heat Capacities 5 5 Q nRT f Ti nRDT 2 2 Equate this expression for Q to the one from our defining equation for molar heat capacity: 5 5 Q nRDT CnDT CP R 2 2 Specific Heat Capacities At constant volume (isochoric): W 0 3 Q CnDT DU W nRDT 2 3 CV R 2 Specific Heat Capacities A couple of things to note: 5 R CP 2 5 CV 3 R 3 2 5 3 CP CV R R R 2 2 (for a monatomic ideal gas) Second Law of Thermodynamics Heat flows spontaneously from regions of higher temperature to regions of lower temperature. It does not flow spontaneously in the other direction. Set your cup of hot coffee down on the sidewalk tonight. Come back and get it in after a few minutes. It won’t be hotter. Second Law and Heat Engines As heat flows from a hotter region to a colder one, a device can be constructed that will use some of that heat to do mechanical work. Such a device is called a heat engine. Heat Engines A familiar example: internal combustion (auto) engine. Hot reservoir: burning fuel-air mixture Cold reservoir: exhaust gases Heat Engines: Energy Conservation The principle of energy conservation requires: QH W QC Heat Engines: Efficiency Efficiency is defined as the ratio of the work done by the heat engine to the input heat it receives. W QH QC QC e 1 QH QH QH Efficiency is a dimensionless, unitless ratio. Efficiency and Reversibility A process is called reversible if both the system and its environment can be returned, after the process, to exactly the same states they were in before the process. irreversible reversible Efficiency and Reversibility No process involving friction is reversible. Also, any process in which heat flows spontaneously from a hot to a cold reservoir is irreversible. The system can be restored to its original state, but the work required changes the environment further from its original state. Carnot’s Principle No irreversible heat engine operating between two reservoirs at constant temperatures can be more efficient than a reversible engine operating between the same temperatures. All reversible engines operating between the same two temperatures have the same efficiency. Sadi Nicolas Leonard Carnot 1796 – 1832 French military engineer Efficiency of a Carnot Engine If a thermodynamic temperature scale is correctly defined, the ratio of the heat into the cold reservoir to the heat from the hot reservoir is equal to the ratio of the reservoir temperatures: QC TC QH TH Lord Kelvin defined his thermodynamic temperature scale so that this is true. The temperatures in the above equation must be absolute (in Kelvins). Efficiency of a Carnot Engine Earlier, we said that the efficiency of a heat engine is QC e 1 QH TC Substituting from the previous equation: eC 1 TH This is true for a Carnot engine. Notice that we are assuming that the reservoir temperaures are not changed by the operation of the engine. A Different Kind of Reversibility A heat engine diverts some of the heat flowing spontaneously from hot to cold, and uses it to generate output work. If we are willing to input work, then we can cause heat to flow from cold to hot. (Heat pump, refrigerator.) Notice that this reverse operation is not the same thing as thermodynamic reversibility. Heat Pumps and Refrigerators Conservation of energy applies to heat pumps as well as to heat engines: QH = W + QC For a thermodynamically-reversible heat pump or refrigerator: QC TC QH TH Refrigerator: coefficient of performance QC coeff. of performanc e W Entropy Entropy: the loss of our ability to use heat to perform work, because of the irreversible spontaneous flow of heat from higher to lower temperatures. If heat flows into or out of a system reversibly, the system’s change in entropy is Q DS T SI units: J / K Entropy and Reversible Engines The change in entropy associated with the operation of a Carnot engine: hot reservoir QH DS H TH cold reservoir QC DSC TC QC QH QC QH total change: DStotal TC TH TC TH Entropy and Reversible Engines But Carnot’s principle said that QC TC QC QH QH TH TC TH So, the total change in entropy associated with the operation of a Carnot engine is QC QH QC QC DStotal 0 TC TH TC TC So: the operation of a reversible engine does not change the total entropy of the universe. Irreversible processes increase the entropy of the universe. Energy Unavailable for Doing Work Consider a reversible engine working between reservoir temperatures of 650K and 150K. Its efficiency: TC 150 K eC 1 1 0.769 TH 650 K If 1200 J of heat are drawn from the hot reservoir, the work is W eQH 0.7691200 J 923 J Energy Unavailable for Doing Work Now we provide a path for our 1200 J of heat to flow irreversibly from the 650K reservoir to a cooler one, at 350K. Now our engine’s efficiency becomes: TC 150 K eC 1 1 0.571 TH 350 K Energy Unavailable for Doing Work At this decreased efficiency, the work done by this 1200 J of heat is: W eQH 0.571 1200 J 685 J The work done by this heat in descending from 650 K to 150 K has decreased by 923 J – 685 J, or 238 J. Energy Unavailable for Doing Work We can also calculate this loss of work by calculating the increase in the entropy of the universe associated with the irreversible part of the heat flow: Q350K Q650K DSuniverse 350 K 650 K 1200 J 1200 J 1.582 J/K 350 K 650 K Energy Unavailable for Doing Work Then apply Eq. 15.19 from your textbook to calculate the energy unavailable for work: Wunavailable T0 DSuniverse Wunavailable (150 K)1.582 J/K 237 J The irreversible flow causes this.