* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 15 Lesson 2
Hypothermia wikipedia , lookup
Thermal conductivity wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
First law of thermodynamics wikipedia , lookup
Temperature wikipedia , lookup
Dynamic insulation wikipedia , lookup
Thermal radiation wikipedia , lookup
Calorimetry wikipedia , lookup
Heat exchanger wikipedia , lookup
Heat transfer physics wikipedia , lookup
Thermoregulation wikipedia , lookup
Heat capacity wikipedia , lookup
R-value (insulation) wikipedia , lookup
Copper in heat exchangers wikipedia , lookup
Heat equation wikipedia , lookup
Thermodynamic system wikipedia , lookup
Countercurrent exchange wikipedia , lookup
Heat transfer wikipedia , lookup
Adiabatic process wikipedia , lookup
Thermal conduction wikipedia , lookup
Second law of thermodynamics wikipedia , lookup
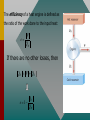
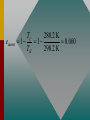
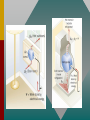
Chapter 15 Lesson 2 THE ZEROTH LAW OF THERMODYNAMICS Two systems individually in thermal equilibrium with a third system are in thermal equilibrium with each other. MOLAR HEAT CAPACITY The molar heat capacity C is defined as the heat per unit mole per Celsius degree. Check with your instructor to see if this more thorough treatment of thermodynamic processes is required. SPECIFIC HEAT CAPACITY Remember the definition of specific heat capacity as the heat per unit mass required to change the temperature? Q c m t For example, copper: c = 390 J/kgK MOLAR SPECIFIC HEAT CAPACITY The “mole” is a better reference for gases than is the “kilogram.” Thus the molar specific heat capacity is defined by: C= Q n T For example, a constant volume of oxygen requires 21.1 J to raise the temperature of one mole by one kelvin degree. SPECIFIC HEAT CAPACITY CONSTANT VOLUME How much heat is required to raise the temperature of 2 moles of O2 from 0oC to 100oC? Q = nCv T Q = (2 mol)(21.1 J/mol K)(373 K - 273 K) Q = +4220 J SPECIFIC HEAT CAPACITY CONSTANT VOLUME (Cont.) Since the volume has not changed, no work is done. The entire 4220 J goes to increase the internal energy, U. Q = U = nCv T = 4220 J U = nCv T Thus, U is determined by the change of temperature and the specific heat at constant volume. SPECIFIC HEAT CAPACITY CONSTANT PRESSURE We have just seen that 4220 J of heat were needed at constant volume. Suppose we want to also do 1000 J of work at constant pressure? Q = U + W Q = 4220 J + J Q = 5220 J Same HEAT CAPACITY (Cont.) Heat to raise temperature of an ideal gas, U, is the same for any process. U = nCvT For constant pressure Q = U + W nCpT = nCvT + P V Cp > Cv Cp Cv REMEMBER, FOR ANY PROCESS INVOLVING AN IDEAL GAS: PV = nRT Q = U + W PAVA TA = PBVB TB U = nCv T The second law is a statement about the natural tendency of heat to flow from hot to cold, whereas the first law deals with energy Conservation and focuses on both heat and work. THE SECOND LAW OF THERMODYNAMICS: THE HEAT FLOW STATEMENT Heat flows spontaneously from a substance at a higher temperature to a substance at a lower temperature and does not flow spontaneously in the reverse direction. A heat engine is any device that uses heat to perform work. It has three essential features. 1. Heat is supplied to the engine at a relatively high temperature from a place called the hot reservoir. 2. Part of the input heat is used to perform work by the working substance of the engine. 3. The remainder of the input heat is rejected to a place called the cold reservoir. QH magnitude of input heat QC magnitude of rejected heat W magnitude of the work done The efficiency of a heat engine is defined as the ratio of the work done to the input heat: e W QH If there are no other losses, then QH W QC e 1 QC QH Example: An Automobile Engine An automobile engine has an efficiency of 22.0% and produces 2510 J of work. How much heat is rejected by the engine? e W QH W QC QH QH W e QH W QC QH W QC QH W 1 QC W W 1 e e W 1 2510 J 1 8900 J 0.220 e A reversible process is one in which both the system and the environment can be returned to exactly the states they were in before the process occurred. CARNOT’S PRINCIPLE: AN ALTERNATIVE STATEMENT OF THE SECOND LAW OF THERMODYNAMICS No irreversible engine operating between two reservoirs at constant Temperatures can have a greater efficiency than a reversible engine operating between the same temperatures. Furthermore, all reversible engines operating between the same temperatures have the same efficiency. QC QH TC TH The Carnot engine is usefule as an idealized model. All of the heat input originates from a single temperature, and all the rejected heat goes into a cold reservoir at a single temperature. Since the efficiency can only depend on the reservoir temperatures, the ratio of heats can only depend on those temperatures. QC QH e 1 QC QH TC 1 TH TC TH Example 7 A Tropical Ocean as a Heat Engine Water near the surface of a tropical ocean has a temperature of 298.2 K, whereas the water 700 meters beneath the surface has a temperature of 280.2 K. It has been proposed that the warm water be used as the hot reservoir and the cool water as the cold reservoir of a heat engine. Find the maximum possible efficiency for such and engine. ecarnot TC 1 TH ecarnot TC 280.2 K 1 1 0.060 TH 298.2 K Refrigerators, air conditioners, and heat pumps are devices that make heat flow from cold to hot. This is called the refrigeration process. The heat pump uses work to make heat from the wintry outdoors flow into the house. Example 10 A Heat Pump An ideal, or Carnot, heat pump is used to heat a house at 294 K. How much work must the pump do to deliver 3350 J of heat into the house on a day when the outdoor temperature is 273 K? QC QH TC TH TC QC QH TH W QH QC TC W QH 1 TH TC W QH 1 TH For heat pump 273 K 3350 J 1 240 J 294 K Coefficien t of performanc e QH W The greater the ratio of Coefficient of Performance, the better the performance of the heat pump or air conditioner/refrigerator. In general, irreversible processes cause us to lose some, but not necessarily all, of the ability to do work. This partial loss can be expressed in terms of a concept called entropy. Carnot QC engine QH entropy change TC TH QC TC QH TH Q S T R reversible Entropy, like internal energy, is a function of the state of the system. Q S T R Consider the entropy change of a Carnot engine. The entropy of the hot reservoir decreases and the entropy of the cold reservoir increases. S QC TC QH TH 0 Reversible processes do not alter the entropy of the universe. Any irreversible process increases the entropy of the universe. S universe 0 THE SECOND LAW OF THERMODYNAMICS STATED IN TERMS OF ENTROPY The total entropy of the universe does not change when a reversible process occurs and increases when an irreversible process occurs. Wunavailable To Suniverse THE THIRD LAW OF THERMODYNAMICS It is not possible to lower the temperature of any system to absolute zero in a finite number of steps.