Concepts for specific heat
... The results is plotted in Fig. 1. In the case β~ω ≫ 1 (i.e. kB T ≪ ~ω) this becomes vanishingly small, while for β~ω ≪ 1 (i.e. kB T ≫ ~ω) we obtain the classical result kB . One says, that the degree of freedom freezes in around a temperature where kB T = ~ω. As the energies of the phonons for solid ...
... The results is plotted in Fig. 1. In the case β~ω ≫ 1 (i.e. kB T ≪ ~ω) this becomes vanishingly small, while for β~ω ≪ 1 (i.e. kB T ≫ ~ω) we obtain the classical result kB . One says, that the degree of freedom freezes in around a temperature where kB T = ~ω. As the energies of the phonons for solid ...
Lecture 2 - Richard Grotjahn
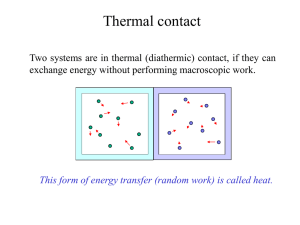
... Heat – some basic concepts part 2 • Heat capacity is amount of energy to raise temperature of object. Different objects need different amounts of heat to raise their temperature a given amount. • Heat felt or measured is “sensible” heat • Heat used to change the state of an object is “latent” heat. ...
... Heat – some basic concepts part 2 • Heat capacity is amount of energy to raise temperature of object. Different objects need different amounts of heat to raise their temperature a given amount. • Heat felt or measured is “sensible” heat • Heat used to change the state of an object is “latent” heat. ...
6.1-6.3 Heat, The Nature of Energy and The First Law of
... • Potential energy – the energy associated with the position or composition of an object. • Kinetic energy – the energy associated with the motion of an object. • Thermal energy – the energy associated with the temperature of an object. • Chemical energy – the energy associated with the relative pos ...
... • Potential energy – the energy associated with the position or composition of an object. • Kinetic energy – the energy associated with the motion of an object. • Thermal energy – the energy associated with the temperature of an object. • Chemical energy – the energy associated with the relative pos ...
Notes in pdf format
... Example: Isobaric expansion of water One gram of water is placed in a cylinder, and the pressure is maintained at 2.0 x 105 Pa. The temperature is raised by 31 C. In one case, the water is in the liquid phase and expands by the small amount of 1.0 x 10-8 m3. In another case, the water is in the gas ...
... Example: Isobaric expansion of water One gram of water is placed in a cylinder, and the pressure is maintained at 2.0 x 105 Pa. The temperature is raised by 31 C. In one case, the water is in the liquid phase and expands by the small amount of 1.0 x 10-8 m3. In another case, the water is in the gas ...
BR. HMWK 2012-03-07 11052
... The internal energy of both containers is identical. The internal energy of container A is half the internal energy of container B. The internal energy of container A is twice the internal energy of container B. The internal energy of container A is more than twice the internal energy of container B ...
... The internal energy of both containers is identical. The internal energy of container A is half the internal energy of container B. The internal energy of container A is twice the internal energy of container B. The internal energy of container A is more than twice the internal energy of container B ...
PPT
... No process is possible whose sole result is the transfer of heat from a body of lower temperature to a body of higher temperature The second law of thermodynamics is an expression of the tendency that over time, differences in temperature, pressure, and chemical potential equilibrate in an isolated ...
... No process is possible whose sole result is the transfer of heat from a body of lower temperature to a body of higher temperature The second law of thermodynamics is an expression of the tendency that over time, differences in temperature, pressure, and chemical potential equilibrate in an isolated ...
Chapter 9: Thermodynamic Processes and Thermochemistry
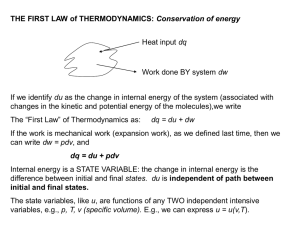
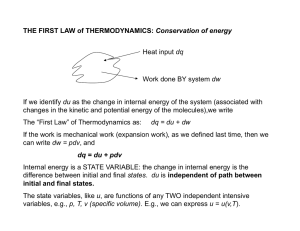
... will differ. Energy change is independent of the pathway, whereas work and heat are both dependent on the pathway. A property that is independent of the pathway is called a state function . Energy is a state function. Work and heat are not state functions. ...
... will differ. Energy change is independent of the pathway, whereas work and heat are both dependent on the pathway. A property that is independent of the pathway is called a state function . Energy is a state function. Work and heat are not state functions. ...
Bagian 2 termodinamika
... collection of matter which has distinct boundaries. OR A real or imaginary portion of universe whish has distinct boundaries is called system. OR A thermodynamic system is that part of universe which is under thermodynamic study. ...
... collection of matter which has distinct boundaries. OR A real or imaginary portion of universe whish has distinct boundaries is called system. OR A thermodynamic system is that part of universe which is under thermodynamic study. ...
Energy, Work and Heat - abuad lms
... The name thermodynamics is derived from the Greek words “therme” meaning heat and dynamis meaning power or motion. Thermodynamics essentially means heat power or heat-inmotion. The oxford Dictionary defines thermodynamics as the science of relation between heat and mechanical energy. It is the study ...
... The name thermodynamics is derived from the Greek words “therme” meaning heat and dynamis meaning power or motion. Thermodynamics essentially means heat power or heat-inmotion. The oxford Dictionary defines thermodynamics as the science of relation between heat and mechanical energy. It is the study ...
Introduction to Thermodynamics
... Work, Heat and Energy Energy: Capacity to do work - the energy of a system can be changed by work and heat Units : joules (J) or for molar energy kJ mol-1 Work : A form of energy which can transfer in and out of a system, that is stored in the organized motion of molecules. Work is done when an obj ...
... Work, Heat and Energy Energy: Capacity to do work - the energy of a system can be changed by work and heat Units : joules (J) or for molar energy kJ mol-1 Work : A form of energy which can transfer in and out of a system, that is stored in the organized motion of molecules. Work is done when an obj ...
THE FIRST LAW of THERMODYNAMICS: Conservation of energy
... No process is possible whose sole result is the transfer of heat from a body of lower temperature to a body of higher temperature The second law of thermodynamics is an expression of the tendency that over time, differences in temperature, pressure, and chemical potential equilibrate in an isolated ...
... No process is possible whose sole result is the transfer of heat from a body of lower temperature to a body of higher temperature The second law of thermodynamics is an expression of the tendency that over time, differences in temperature, pressure, and chemical potential equilibrate in an isolated ...
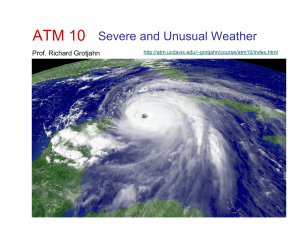
Energy and Radiation Reading: p. 25
... The potential energy stored in the vertical temperature and moisture profiles is released in the form of strong storm updrafts (kinetic energy). We’ll later quantify the above concept as CAPE: Convective Available Potential Energy Note in the previous two examples that the potential energy was conve ...
... The potential energy stored in the vertical temperature and moisture profiles is released in the form of strong storm updrafts (kinetic energy). We’ll later quantify the above concept as CAPE: Convective Available Potential Energy Note in the previous two examples that the potential energy was conve ...