HeatTransfer
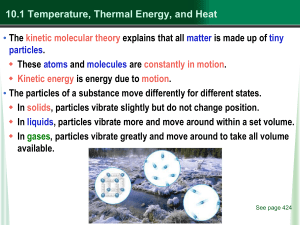
... Thermal Energy Transfer • Conduction is the transfer of heat by direct contact. Heat moves from higher temperature (higher kinetic energy) particles to lower temperature particles (lower kinetic energy.) e.g.: a cold spoon warms when placed in a cup of hot coffee. Thermal conductors transfer ...
... Thermal Energy Transfer • Conduction is the transfer of heat by direct contact. Heat moves from higher temperature (higher kinetic energy) particles to lower temperature particles (lower kinetic energy.) e.g.: a cold spoon warms when placed in a cup of hot coffee. Thermal conductors transfer ...
PY2104 - Introduction to thermodynamics and Statistical physics
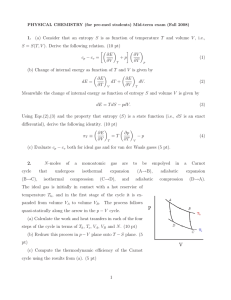
... where n is the number of moles, R is the universal gas constant, U is the internal energy, V is the volume and σ is constant. Calculae the specific heats at constant pressure and constant volume, cp and cv for this gas. 2) (i) Show that for a perfect gas undergoing adiabatic expansion, pV γ is const ...
... where n is the number of moles, R is the universal gas constant, U is the internal energy, V is the volume and σ is constant. Calculae the specific heats at constant pressure and constant volume, cp and cv for this gas. 2) (i) Show that for a perfect gas undergoing adiabatic expansion, pV γ is const ...
Name
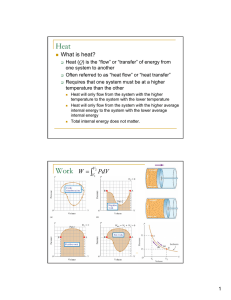
... The work done on the gas is negative When the volume remains constant No work is done on the gas The pressure remains constant during the expansion or compression This is called an isobaric process If the pressure changes, the average pressure may be used to estimate the work done Used ...
... The work done on the gas is negative When the volume remains constant No work is done on the gas The pressure remains constant during the expansion or compression This is called an isobaric process If the pressure changes, the average pressure may be used to estimate the work done Used ...
Summary presentation 10.2 File
... is to do work on the system or allow work to be done by the system on the surroundings. ...
... is to do work on the system or allow work to be done by the system on the surroundings. ...
ENT 211 Tutorial Week 1
... Why is Heat Transfer a nonequilibrium phenomenon? Heat transfer is a non-equilibrium phenomena since in a system that is in equilibrium there can be no temperature differences and thus no heat flow. ...
... Why is Heat Transfer a nonequilibrium phenomenon? Heat transfer is a non-equilibrium phenomena since in a system that is in equilibrium there can be no temperature differences and thus no heat flow. ...
Heat Work
... When temperature changes, internal energy has changed – may happen through heat transfer or through mechanical work First law is a statement of conservation of energy Change in internal energy of system equals the difference between the heat added to the system and the work done by the system ΔU = Q ...
... When temperature changes, internal energy has changed – may happen through heat transfer or through mechanical work First law is a statement of conservation of energy Change in internal energy of system equals the difference between the heat added to the system and the work done by the system ΔU = Q ...
Text Questions
... 53. By “standard conditions,” we usually mean a pressure of ______ and a temperature of _____. 54. How do we define standard enthalpy change? ...
... 53. By “standard conditions,” we usually mean a pressure of ______ and a temperature of _____. 54. How do we define standard enthalpy change? ...