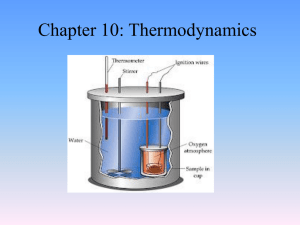
Heat Chapter 12: Thermodynamics
... • Heat does not flow spontaneously from a colder to a warmer body. • In a thermal cycle, heat energy cannot be completely transformed into mechanical work. • The total entropy of the universe increases in every natural process. The Third Law of Thermodynamics – It is not possible to lower temperatur ...
... • Heat does not flow spontaneously from a colder to a warmer body. • In a thermal cycle, heat energy cannot be completely transformed into mechanical work. • The total entropy of the universe increases in every natural process. The Third Law of Thermodynamics – It is not possible to lower temperatur ...
State Variables
... evaluated between the initial and final states – This is true whether or not the pressure ...
... evaluated between the initial and final states – This is true whether or not the pressure ...
Lacture №1. Chemical thermodynamics. The first law of
... If the gas expands, V2 › V1 and work is done by the system and W is negative (we will use sign positive) V2‹ V1 and the work is done on the system and W is positive Note. It may be noted that many books use the opposite sign convention for work!!! (according to the IUPAC recommendation) ...
... If the gas expands, V2 › V1 and work is done by the system and W is negative (we will use sign positive) V2‹ V1 and the work is done on the system and W is positive Note. It may be noted that many books use the opposite sign convention for work!!! (according to the IUPAC recommendation) ...
The Second Law of Thermodynamics
... The problems arise from: 1.Classical thermodynamics is connected with states of equilibrium and various processes connecting them. 2.The exact process by which a system reaches the final state from its initial state is immaterial. i.e. the transition is independent of the particular path taken 3. T ...
... The problems arise from: 1.Classical thermodynamics is connected with states of equilibrium and various processes connecting them. 2.The exact process by which a system reaches the final state from its initial state is immaterial. i.e. the transition is independent of the particular path taken 3. T ...
Atomic Structure
... 1. Consider the human body as a system and apply the first law of thermodynamics to it. We know that over any given period of sufficient length (say one day), there will be a net heat flow from the body (i.e. Q is negative) and the body will do some external work on its surroundings (i.e. W is posit ...
... 1. Consider the human body as a system and apply the first law of thermodynamics to it. We know that over any given period of sufficient length (say one day), there will be a net heat flow from the body (i.e. Q is negative) and the body will do some external work on its surroundings (i.e. W is posit ...
Thermochemistry
... • calorie(c)-amount of energy required to raise the temperature of one gram of water by one Celsius degree. (Food you eat is measured in Kilocalories which is abbreviated C). • Joule (J)-the SI unit of energy • 1 c=4.184J ...
... • calorie(c)-amount of energy required to raise the temperature of one gram of water by one Celsius degree. (Food you eat is measured in Kilocalories which is abbreviated C). • Joule (J)-the SI unit of energy • 1 c=4.184J ...
Chapter Summary
... A cycle is a sequence of processes that returns a system to its original state. The cycle as a whole satisfies the first law of thermodynamics, as does each of its processes. The change in internal energy for any cycle is always zero, because the system returns to its initial state, and the area of ...
... A cycle is a sequence of processes that returns a system to its original state. The cycle as a whole satisfies the first law of thermodynamics, as does each of its processes. The change in internal energy for any cycle is always zero, because the system returns to its initial state, and the area of ...
Topic 2 The first law of thermodynamics
... If A and C are each in thermal equilibrium with B, A is also in equilibrium with C. Temperature as a quality of heat, by Galileo and Newton The temperatures are equal for all systems in thermal equilibrium. Temperature scale Thermometers ...
... If A and C are each in thermal equilibrium with B, A is also in equilibrium with C. Temperature as a quality of heat, by Galileo and Newton The temperatures are equal for all systems in thermal equilibrium. Temperature scale Thermometers ...
Vocabulary
... gases within the cylinders of an automobile engine is nearly adiabatic. __TRUE TRUE__ 8. What happens to a gas when it adiabatically expands and does work on its surroundings? It loses internal energy and cools down 9. Circle the letter that describes the adiabatic form of the first law of thermodyn ...
... gases within the cylinders of an automobile engine is nearly adiabatic. __TRUE TRUE__ 8. What happens to a gas when it adiabatically expands and does work on its surroundings? It loses internal energy and cools down 9. Circle the letter that describes the adiabatic form of the first law of thermodyn ...
Chapter 15 Notes - Valdosta State University
... type of wall. A wall that allows heat flow is called a diathermal wall. One that does not is said to be adiabatic. The state of a system is described by specifying factors that affect the internal energy of the system. In the case of a gas this would be temperature, pressure, volume, and mass. There ...
... type of wall. A wall that allows heat flow is called a diathermal wall. One that does not is said to be adiabatic. The state of a system is described by specifying factors that affect the internal energy of the system. In the case of a gas this would be temperature, pressure, volume, and mass. There ...
Atomic Structure
... broken down and oxidized (has oxygen added), and there is energy released. c. We gain energy from the food, air and water that we take in. This energy is converted to heat and into work, and stored as potential energy, for example in fats. The total energy is always conserved, and the change in inte ...
... broken down and oxidized (has oxygen added), and there is energy released. c. We gain energy from the food, air and water that we take in. This energy is converted to heat and into work, and stored as potential energy, for example in fats. The total energy is always conserved, and the change in inte ...
Lecture 3 - McMaster Physics and Astronomy
... in any way that is convenient. Fourth, heat is not a property of a system like temperature, pressure, volume or mass. It is energy in transit – energy that enters or leaves a system as a consequence of a temperature difference between the system and a body with which it is in thermal contact (includ ...
... in any way that is convenient. Fourth, heat is not a property of a system like temperature, pressure, volume or mass. It is energy in transit – energy that enters or leaves a system as a consequence of a temperature difference between the system and a body with which it is in thermal contact (includ ...
název projektu
... violates the first law of thermodynamics: the law of conservation of energy. •A perpetual motion machine of the second kind is a machine which spontaneously converts thermal energy into mechanical work. When the thermal energy is equivalent to the work done, this does not violate the law of conserva ...
... violates the first law of thermodynamics: the law of conservation of energy. •A perpetual motion machine of the second kind is a machine which spontaneously converts thermal energy into mechanical work. When the thermal energy is equivalent to the work done, this does not violate the law of conserva ...