15.1,2
... C H A P T E R 15 Thermodynamics Thermodynamics is the branch of physics that is built upon the fundamental laws that heat and work obey. ...
... C H A P T E R 15 Thermodynamics Thermodynamics is the branch of physics that is built upon the fundamental laws that heat and work obey. ...
Themodynamic notes section 6.1
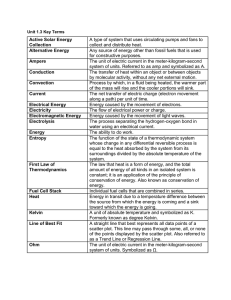
... • The entropy of a system not in thermal equilibrium will increase. • The entropy of a system approaches a constant value as the system approaches absolute zero. ...
... • The entropy of a system not in thermal equilibrium will increase. • The entropy of a system approaches a constant value as the system approaches absolute zero. ...
Section 11
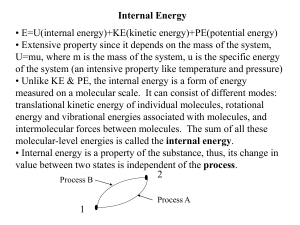
... Work can transfer energy to a substance which increases the internal energy of the substance. Internal energy can then decrease through the transfer of energy as heat. Energy can be transferred to a substance as heat and from the substance as work. Internal energy of a substance has been treated ...
... Work can transfer energy to a substance which increases the internal energy of the substance. Internal energy can then decrease through the transfer of energy as heat. Energy can be transferred to a substance as heat and from the substance as work. Internal energy of a substance has been treated ...
Chapter 1: The first law of thermodynamics
... and volume we say that the quantity is a function of state. Therefore, for an ideal gas in equilibrium, the system’s temperature is a function of state ( θ = F ( P,V ) ). A quantity, dG, is said to be an exact differential if it only depends on the difference in the function of state between two clo ...
... and volume we say that the quantity is a function of state. Therefore, for an ideal gas in equilibrium, the system’s temperature is a function of state ( θ = F ( P,V ) ). A quantity, dG, is said to be an exact differential if it only depends on the difference in the function of state between two clo ...
Thermodynamic principles. - med.muni
... states with no change in the system or surroundings. • Irreversible process • Cyclic process: the initial and final states of the system are identical (but not necessarily the surroundings) • Sign convention: energy given to a system and work done by an external force on the system are considered to ...
... states with no change in the system or surroundings. • Irreversible process • Cyclic process: the initial and final states of the system are identical (but not necessarily the surroundings) • Sign convention: energy given to a system and work done by an external force on the system are considered to ...
Relationships Between Heat and Work
... • Internal energy is constant in a constanttemperature process • Isothermal process – a thermodynamic process that takes place at constant temperature and in which the internal energy of a system remains unchanged – Similar to a balloon expanding as the pressure drops before a storm hits • The ballo ...
... • Internal energy is constant in a constanttemperature process • Isothermal process – a thermodynamic process that takes place at constant temperature and in which the internal energy of a system remains unchanged – Similar to a balloon expanding as the pressure drops before a storm hits • The ballo ...
Verdana 30 pt
... when mechanical work is transformed entirely into heat, the relationship between work, measured in joules, and heat, expressed in calories, remains constant in any process. ...
... when mechanical work is transformed entirely into heat, the relationship between work, measured in joules, and heat, expressed in calories, remains constant in any process. ...