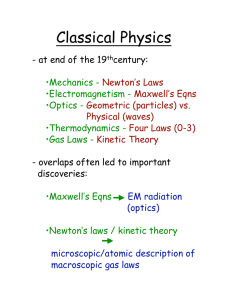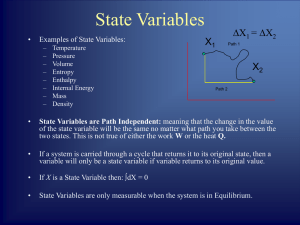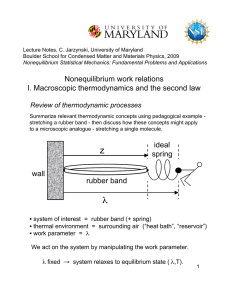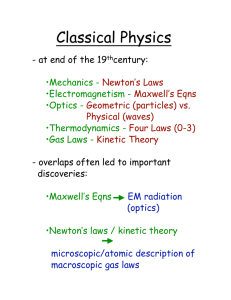
6. Absorption of Heat
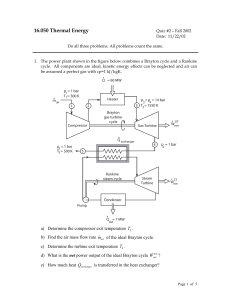
... HRW 75E (5th ed.). Gas within a chamber passes through the cycle shown in Fig. 19-37. Determine the net heat added to the system during process CA if the heat QAB added during process AB is 20.0 J, no heat is transferred during process BC, and the net work dome during the cycle is 15.0 J. Since the ...
... HRW 75E (5th ed.). Gas within a chamber passes through the cycle shown in Fig. 19-37. Determine the net heat added to the system during process CA if the heat QAB added during process AB is 20.0 J, no heat is transferred during process BC, and the net work dome during the cycle is 15.0 J. Since the ...
Thermodynamics
... process, what is the total amount of energy transferred as heat? Has energy been added to or removed from the refrigerant as heat? ...
... process, what is the total amount of energy transferred as heat? Has energy been added to or removed from the refrigerant as heat? ...
Ionic Equations
... Energy • A roller coaster at the top of a hill has a great amount of potential energy. • As the rollercoaster begins to speed down the hill, the potential energy is turned into kinetic energy ...
... Energy • A roller coaster at the top of a hill has a great amount of potential energy. • As the rollercoaster begins to speed down the hill, the potential energy is turned into kinetic energy ...
solutions
... • Zeroth: If two systems are both in thermal equilibrium with a third then they are in thermal equilibrium with each other. • First: The increase in internal energy of a closed system is equal to the heat supplied to the system minus work done by it. • Second: The entropy of any isolated system neve ...
... • Zeroth: If two systems are both in thermal equilibrium with a third then they are in thermal equilibrium with each other. • First: The increase in internal energy of a closed system is equal to the heat supplied to the system minus work done by it. • Second: The entropy of any isolated system neve ...
First law of thermodynamics
... or lost as the result of work". Internal energy is a property of the system whereas work done and heat supplied are not. A significant result of this distinction is that a given internal energy change ΔU can be achieved by, in principle, many combinations of heat and work. ...
... or lost as the result of work". Internal energy is a property of the system whereas work done and heat supplied are not. A significant result of this distinction is that a given internal energy change ΔU can be achieved by, in principle, many combinations of heat and work. ...
here
... If A and B are each in thermal equilibrium with a third body C, then A and B are in thermal equilibrium. Thermal equilibrium means that two bodies are in states such that if they are connected, then their condition will not change. ...
... If A and B are each in thermal equilibrium with a third body C, then A and B are in thermal equilibrium. Thermal equilibrium means that two bodies are in states such that if they are connected, then their condition will not change. ...
2-Basic Concepts of Thermodynamics
... 1.1 Thermodynamics and Energy Definition Science that deals with heat and work and the changes they can produce. e.g. change of temperature (T), pressure (P) etc. Basis is experimental observations written down as laws. e.g. 1st law of thermodynamics: Energy can change from one form to another but t ...
... 1.1 Thermodynamics and Energy Definition Science that deals with heat and work and the changes they can produce. e.g. change of temperature (T), pressure (P) etc. Basis is experimental observations written down as laws. e.g. 1st law of thermodynamics: Energy can change from one form to another but t ...
W - Boulder School for Condensed Matter and Materials Physics
... • thermal environment = surrounding air (“heat bath”, “reservoir”) • work parameter = λ We act on the system by manipulating the work parameter. λ fixed → system relaxes to equilibrium state ( λ,T). ...
... • thermal environment = surrounding air (“heat bath”, “reservoir”) • work parameter = λ We act on the system by manipulating the work parameter. λ fixed → system relaxes to equilibrium state ( λ,T). ...
The Thermodynamic Potentials
... Thus, the removal of a constraint in each case results in the onset of some spontaneous process, and when the systems finally settle into new equilibrium states they do so with the new values of the parameters U(1), V(1), N1(1) … and U(2), V(2), N1(2) … . The basic problem of thermodynamics is the c ...
... Thus, the removal of a constraint in each case results in the onset of some spontaneous process, and when the systems finally settle into new equilibrium states they do so with the new values of the parameters U(1), V(1), N1(1) … and U(2), V(2), N1(2) … . The basic problem of thermodynamics is the c ...
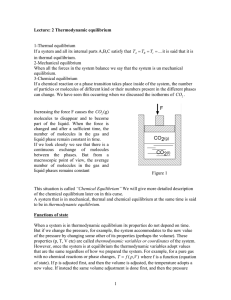
1 Lecture: 2 Thermodynamic equilibrium 1
... constant for a conservative system. If we include all the variables that describe the processes, all systems are conservative. It follows that the energy is always conserved. We consider a system “A” surrounded by the rest of the universe, and we say that the system has a certain amount of energy U ...
... constant for a conservative system. If we include all the variables that describe the processes, all systems are conservative. It follows that the energy is always conserved. We consider a system “A” surrounded by the rest of the universe, and we say that the system has a certain amount of energy U ...
• Thermodynamics, what is it? • System, Surrounding and Boundary
... A pure substance can exist in more than one phase, but its chemical composition must be the same in each phase. For example, if liquid water and water vapor form a system with two phases, the system can be regarded as a pure substance because each phase has the same composition. The nature of phases ...
... A pure substance can exist in more than one phase, but its chemical composition must be the same in each phase. For example, if liquid water and water vapor form a system with two phases, the system can be regarded as a pure substance because each phase has the same composition. The nature of phases ...
Thermochemistry - all things chemistry with dr. cody
... Calculate the enthalpy change for the hydrogenation of ethylene using the following thermochemical equations: C2H4(g) + H2(g) → C2H6(g) H = ? H2(g) + ½ O2(g) → H2O(l) C2H4(g) + 3 O2(g) → 2 CO2(g) + 2 H2O(l) C2H6(g) + 7/2 O2(g) → 2CO2(g) + 3 H2O(l) ...
... Calculate the enthalpy change for the hydrogenation of ethylene using the following thermochemical equations: C2H4(g) + H2(g) → C2H6(g) H = ? H2(g) + ½ O2(g) → H2O(l) C2H4(g) + 3 O2(g) → 2 CO2(g) + 2 H2O(l) C2H6(g) + 7/2 O2(g) → 2CO2(g) + 3 H2O(l) ...