Thermodynamics
... A state variable describes the state of a system at time t, but it does not reveal how the system was put into that state. Examples of state variables: pressure, temperature, volume, number of moles, and internal energy. Thermal processes can change the state of a system. We assume that thermal proc ...
... A state variable describes the state of a system at time t, but it does not reveal how the system was put into that state. Examples of state variables: pressure, temperature, volume, number of moles, and internal energy. Thermal processes can change the state of a system. We assume that thermal proc ...
Set 3
... In such a case, it makes sense to write down a formula for the work done for, say an Ideal Gas. This is one way to compare our models for the internal energies of the system. ...
... In such a case, it makes sense to write down a formula for the work done for, say an Ideal Gas. This is one way to compare our models for the internal energies of the system. ...
Application , First, Law of Thermodynamics
... a. The change in internal energy of a system is solely due to the work done on the system. Hence, there is zero energy transfer by heat. i. Such a process occurs when the system is thermally isolated. ii. It also depends on the brevity of the process (i.e., a very fast thermodynamic process can be c ...
... a. The change in internal energy of a system is solely due to the work done on the system. Hence, there is zero energy transfer by heat. i. Such a process occurs when the system is thermally isolated. ii. It also depends on the brevity of the process (i.e., a very fast thermodynamic process can be c ...
Solution Tutorial 4 - Aerospace Engineering, IIT Madras
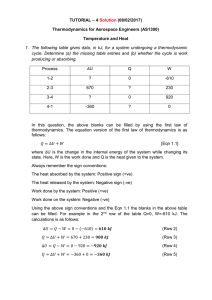
... 6. 4 Kg of air is contained in a vertical piston cylinder with the top open to atmosphere. The piston weighs 50 kg and has a face area of 0.01 m2. The air initially occupies a volume of 0.005 m3 . The air now undergoes a process wherein its volume deceases to 0.0025 m3 and 1.41 kJ of heat is lost t ...
... 6. 4 Kg of air is contained in a vertical piston cylinder with the top open to atmosphere. The piston weighs 50 kg and has a face area of 0.01 m2. The air initially occupies a volume of 0.005 m3 . The air now undergoes a process wherein its volume deceases to 0.0025 m3 and 1.41 kJ of heat is lost t ...
Lecture 5
... 47. When a system is taken from state i to state f along path iaf in the figure below, Q = 50 cal and W = 20 cal. Along path ibf, Q = 36 cal. (a) What is W along path ibf? (b) If W = -13 cal for the return path fi, what is Q for this path? (c) If Eint,i = 10 cal, what is Eint,f? If Eint,b = 22 cal, ...
... 47. When a system is taken from state i to state f along path iaf in the figure below, Q = 50 cal and W = 20 cal. Along path ibf, Q = 36 cal. (a) What is W along path ibf? (b) If W = -13 cal for the return path fi, what is Q for this path? (c) If Eint,i = 10 cal, what is Eint,f? If Eint,b = 22 cal, ...
Lecture 1 1 Overview
... We will use V for extensive, Ṽ for molar, V̂ for specific, and V i for partial molar. 3. Gibbs Phase Rule. We observe that there are many variables for a given system, e.g., pressure, density, internal energy, viscosity, thermal conductivity, composition, volume, etc. How many of these properties d ...
... We will use V for extensive, Ṽ for molar, V̂ for specific, and V i for partial molar. 3. Gibbs Phase Rule. We observe that there are many variables for a given system, e.g., pressure, density, internal energy, viscosity, thermal conductivity, composition, volume, etc. How many of these properties d ...
The Second Law of Thermodynamics
... If we adjust the sizes of E and R such that all the work is used to run the Carnot refrigerator, and view the combined E-R apparatus as the system, we have succeeded in creating a device, which spontaneously pumps heat from cold to hot without any work input from the surroundings. This violates Clau ...
... If we adjust the sizes of E and R such that all the work is used to run the Carnot refrigerator, and view the combined E-R apparatus as the system, we have succeeded in creating a device, which spontaneously pumps heat from cold to hot without any work input from the surroundings. This violates Clau ...
lecture21
... Processes proceed in a certain direction and not in the reverse direction. The first law places no restriction on direction. A process will not occur unless it satisfies both the first and second laws of thermodynamics. Second law not only identifies the direction of process, it also asserts that en ...
... Processes proceed in a certain direction and not in the reverse direction. The first law places no restriction on direction. A process will not occur unless it satisfies both the first and second laws of thermodynamics. Second law not only identifies the direction of process, it also asserts that en ...