Chapter 1 Thermodynamics
... To show the equality of the two statements it will be useful to define a heat engine. Heat engine is a system which undergoes a cyclic transformation that takes heat Q1 from a warmer reservoir, converts some of it to work W and rejects the rest Q2 = Q1 ≠ W to a colder reservoir. In contrast, refrige ...
... To show the equality of the two statements it will be useful to define a heat engine. Heat engine is a system which undergoes a cyclic transformation that takes heat Q1 from a warmer reservoir, converts some of it to work W and rejects the rest Q2 = Q1 ≠ W to a colder reservoir. In contrast, refrige ...
ppt - Physics Rocks!
... Thermodynamic Processes are any processes that will result in the change of the state of a system Heating a gas Compressing the gas (doing work TO the gas) Expansion of the gas (work done BY the gas) ...
... Thermodynamic Processes are any processes that will result in the change of the state of a system Heating a gas Compressing the gas (doing work TO the gas) Expansion of the gas (work done BY the gas) ...
Laws of Thermodynamics
... conserved. However, there was a second class of suggested perpetual motion machines which didn’t work but which were consistent with energy conservation. Another law of thermodynamics was introduced to summarize the fact that this class of perpetual motion machines also does not work. This is the se ...
... conserved. However, there was a second class of suggested perpetual motion machines which didn’t work but which were consistent with energy conservation. Another law of thermodynamics was introduced to summarize the fact that this class of perpetual motion machines also does not work. This is the se ...
The First Law of Thermodynamics: Closed Systems Heat Transfer
... The net work or cycle work is shown in Fig. 5. In a cycle, the net change for any properties (point functions or exact differentials) is zero. However, the net work and heat transfer depend on the cycle path. ΔU = ΔP = ΔT = Δ(any property) = 0 for a cycle ...
... The net work or cycle work is shown in Fig. 5. In a cycle, the net change for any properties (point functions or exact differentials) is zero. However, the net work and heat transfer depend on the cycle path. ΔU = ΔP = ΔT = Δ(any property) = 0 for a cycle ...
Unit 6 Review
... 15. A gas undergoes an isobaric process where it experiences an increase in temperature while expanding. Is heat being added or removed during this process? Analyze the first law of thermodynamics to help you answer this question. ...
... 15. A gas undergoes an isobaric process where it experiences an increase in temperature while expanding. Is heat being added or removed during this process? Analyze the first law of thermodynamics to help you answer this question. ...
Energy
... determined by transfer of heat (q) observed by changes in temperature. • First you need to define what your system and surroundings are… • When heat moves out of a system to the surroundings the process is exothermic for the system. • When heat moves into a system from the surroundings the process i ...
... determined by transfer of heat (q) observed by changes in temperature. • First you need to define what your system and surroundings are… • When heat moves out of a system to the surroundings the process is exothermic for the system. • When heat moves into a system from the surroundings the process i ...
Thermodynamics
... Temperature is "a measure of the average kinetic energy of the particles in a sample of matter, expressed in terms of units or degrees designated on a standard scale". The most commonly used temperature scale is Celsius, which is based on the freezing and boiling points of water, assigning respectiv ...
... Temperature is "a measure of the average kinetic energy of the particles in a sample of matter, expressed in terms of units or degrees designated on a standard scale". The most commonly used temperature scale is Celsius, which is based on the freezing and boiling points of water, assigning respectiv ...
Chapter 3. Energy and the First Law
... of the system is preserved. It is a state function. • Work is energy in transit; it appears when energy is passed from one system into another. • Work is not a storable quantity as such. Work that enters a system must be stored in some form of energy. • Work is associated with a direction from one s ...
... of the system is preserved. It is a state function. • Work is energy in transit; it appears when energy is passed from one system into another. • Work is not a storable quantity as such. Work that enters a system must be stored in some form of energy. • Work is associated with a direction from one s ...
lec38 - UConn Physics
... PV diagram This allows us to visualize the process through which the gas is progressing ...
... PV diagram This allows us to visualize the process through which the gas is progressing ...
4.1 Classical Thermodynamics: The First Law
... the conduction of the aforementioned experiment, in particular one knows the internal energy for the material at two given states, 1 and 2. Relax now the condition that the changes are adiabatic. What this means is that if one now brings the material into contact with another body, the properties of ...
... the conduction of the aforementioned experiment, in particular one knows the internal energy for the material at two given states, 1 and 2. Relax now the condition that the changes are adiabatic. What this means is that if one now brings the material into contact with another body, the properties of ...
A note on the variation of specific heats in ideal gases Most diatomic
... Recall that "specific heat" is a somewhat misleading term that dates back to the time when heat was viewed as a fluid that could be held by a material, hence terms like specific heat, heat capacity, etc. Today we know that "heat" and "work" should always be understood as "heat transfer" and "work tr ...
... Recall that "specific heat" is a somewhat misleading term that dates back to the time when heat was viewed as a fluid that could be held by a material, hence terms like specific heat, heat capacity, etc. Today we know that "heat" and "work" should always be understood as "heat transfer" and "work tr ...
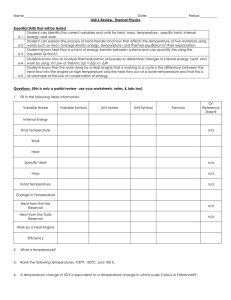
Section 1 – Thermal Energy
... º As a substance absorbs heat its temperature changes depending on the nature of the substance. º Also depends on how much heat is added. º Specific Heat – the amount of heat that is needed to raise the temperature of 1kg of a substance by 1 degree or 1K. º Measured in joules per kilogram Kelvin [J/ ...
... º As a substance absorbs heat its temperature changes depending on the nature of the substance. º Also depends on how much heat is added. º Specific Heat – the amount of heat that is needed to raise the temperature of 1kg of a substance by 1 degree or 1K. º Measured in joules per kilogram Kelvin [J/ ...
Thermodynamics
... • A heat engine is a system that performs the conversion of heat to mechanical work. A heat "source" generates thermal energy that brings the working substance to the high temperature state. The working substance generates work in the "working body" of the engine while transferring heat to the cold ...
... • A heat engine is a system that performs the conversion of heat to mechanical work. A heat "source" generates thermal energy that brings the working substance to the high temperature state. The working substance generates work in the "working body" of the engine while transferring heat to the cold ...
The Laws of Thermodinamics
... The curve on the diagram is called the path taken between the initial and final states The work done depends on the particular path ...
... The curve on the diagram is called the path taken between the initial and final states The work done depends on the particular path ...
Instructor: Hacker Engineering 232 Sample Exam 1 Solutions Answer Key
... his velocity would also have been straight down, and into the ground would go Ricky; but the quarter pipe redirected his motion to being tangent to the ground, while preserving his speed. So instead of busting his head, and winding up dead; Ricky sped off to work, where he was a clerk. Were it not f ...
... his velocity would also have been straight down, and into the ground would go Ricky; but the quarter pipe redirected his motion to being tangent to the ground, while preserving his speed. So instead of busting his head, and winding up dead; Ricky sped off to work, where he was a clerk. Were it not f ...
General Physics (PHY 2130) - Wayne State University Physics and
... • Consider energy conservation in thermal ...
... • Consider energy conservation in thermal ...