Document
... In a system in thermal equilibrium with its surroundings at a temperature T, there is a transfer of energy as heat when a change in the system occurs and the Clausius inequality will read as above: ...
... In a system in thermal equilibrium with its surroundings at a temperature T, there is a transfer of energy as heat when a change in the system occurs and the Clausius inequality will read as above: ...
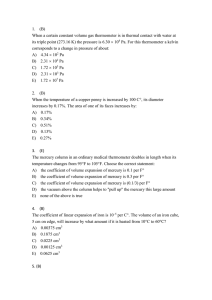
AT620 Review for Midterm #1
... Joule’s law: Internal energy of an ideal gas is a function of temperature only. (this comes from the fact that ideal gas molecules are not attracted to one another) ...
... Joule’s law: Internal energy of an ideal gas is a function of temperature only. (this comes from the fact that ideal gas molecules are not attracted to one another) ...
Quiz_MATH.rtf
... A certain humidifier operates by raising water to the boiling point and then evaporating it. Every minute 30 g of water at 20C are added to replace the 30 g that are evaporated. The heat of fusion of water is333 kJ/kg, the heat of vaporization is 2256 kj/kg, and the specific heat is 4190 J/kg ...
... A certain humidifier operates by raising water to the boiling point and then evaporating it. Every minute 30 g of water at 20C are added to replace the 30 g that are evaporated. The heat of fusion of water is333 kJ/kg, the heat of vaporization is 2256 kj/kg, and the specific heat is 4190 J/kg ...
AP Ch.18 - mrmacphysics
... • C) Is the work from ABC (more, less, or the same) as the work from ADC. • Work is path dependent. ...
... • C) Is the work from ABC (more, less, or the same) as the work from ADC. • Work is path dependent. ...
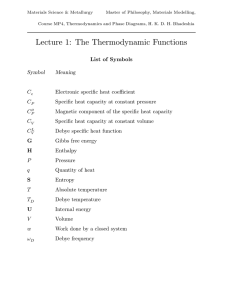
Lecture 6 Free Energy
... It is the tendency of that molecular specie to chemically react, which depends on both its concentration and internal energy of the molecule. In a chemical reaction A ! B , the difference between A and B is amount of chemical energy available to do work per unit of molecule. At chemical equilibrium, ...
... It is the tendency of that molecular specie to chemically react, which depends on both its concentration and internal energy of the molecule. In a chemical reaction A ! B , the difference between A and B is amount of chemical energy available to do work per unit of molecule. At chemical equilibrium, ...
Document
... In a system in thermal equilibrium with its surroundings at a temperature T, there is a transfer of energy as heat when a change in the system occurs and the Clausius inequality will read as above: ...
... In a system in thermal equilibrium with its surroundings at a temperature T, there is a transfer of energy as heat when a change in the system occurs and the Clausius inequality will read as above: ...
Lecture 4
... process has a constant entropy (i.e. isentropic). Isothermal process: Tds = dq, the integrated solution is ∆s = q/T Isothermal and isobaric change of phase: ∆s = l/T. is the latent heat of transformation. Isochoric process: Since Tds = dq = du + PdV, then Tds = du =cv dT, then ∆s = cv ln(T2/T1) if c ...
... process has a constant entropy (i.e. isentropic). Isothermal process: Tds = dq, the integrated solution is ∆s = q/T Isothermal and isobaric change of phase: ∆s = l/T. is the latent heat of transformation. Isochoric process: Since Tds = dq = du + PdV, then Tds = du =cv dT, then ∆s = cv ln(T2/T1) if c ...
1 11.8 Definition of entropy and the modern statement of the second
... initial value. To restore the initial state of the gas without causing any net change in the equilibrium states of the systems involved in this compression, we must make sure: (i) to decrease the temperature of the gas back to its original value; (ii) to move the piston back to its original position ...
... initial value. To restore the initial state of the gas without causing any net change in the equilibrium states of the systems involved in this compression, we must make sure: (i) to decrease the temperature of the gas back to its original value; (ii) to move the piston back to its original position ...
Practice Exam
... a. All of the liquid will eventually boil off leaving nothing but vapor in the vessel. b. The pressure will increase according to Pv = RT c. If the pressure increases to a high enough value there will be no difference between liquid and solid. d. The liquid level in the tank will eventually rise to ...
... a. All of the liquid will eventually boil off leaving nothing but vapor in the vessel. b. The pressure will increase according to Pv = RT c. If the pressure increases to a high enough value there will be no difference between liquid and solid. d. The liquid level in the tank will eventually rise to ...
any physical system, whether or not it can exchange energy and
... can be described by specifying its properties, such as pressure, temperature, or chemical composition. If the external constraints are changed, then these properties will generally alter. The science of thermodynamics attempts to describe mathematically these changes and to predict the equilibrium c ...
... can be described by specifying its properties, such as pressure, temperature, or chemical composition. If the external constraints are changed, then these properties will generally alter. The science of thermodynamics attempts to describe mathematically these changes and to predict the equilibrium c ...
Carnot - UniMAP Portal
... • Steady state is a more general situation than Dynamic equilibrium. If a system is in steady state then the recently observed behaviors of the system will continue into the future. In stochastic systems, the probabilities that various different states will be repeated will remain constant. • In man ...
... • Steady state is a more general situation than Dynamic equilibrium. If a system is in steady state then the recently observed behaviors of the system will continue into the future. In stochastic systems, the probabilities that various different states will be repeated will remain constant. • In man ...
Thermodynamic system
... Thermodynamic equilibrium • A system in thermodynamic equilibrium would remain in the same state if we isolate it. There are no net fluxes. • System is in thermodynamic equilibrium with environment if it has same T (thermal eq.), same p (mechanical eq.), same chemical potential (chemical eq.), and ...
... Thermodynamic equilibrium • A system in thermodynamic equilibrium would remain in the same state if we isolate it. There are no net fluxes. • System is in thermodynamic equilibrium with environment if it has same T (thermal eq.), same p (mechanical eq.), same chemical potential (chemical eq.), and ...
Study Guide Thermodynamics
... Two bodies are in rotational equilibrium if they are at the same temperature. ...
... Two bodies are in rotational equilibrium if they are at the same temperature. ...
2nd law of thermodynamics
... • Isolated system is a system which neither can exchange mass nor energy with the surrounding. • Closed system is a system which can exchange energy but not mass with surroundings. • Open system is a system which can exchange matter as well as energy with the surroundings. Homogeneous system - all o ...
... • Isolated system is a system which neither can exchange mass nor energy with the surrounding. • Closed system is a system which can exchange energy but not mass with surroundings. • Open system is a system which can exchange matter as well as energy with the surroundings. Homogeneous system - all o ...